Press release no. 661 – L’Agenzia Italiana del Farmaco ha pubblicato l’ottavo Rapporto di 
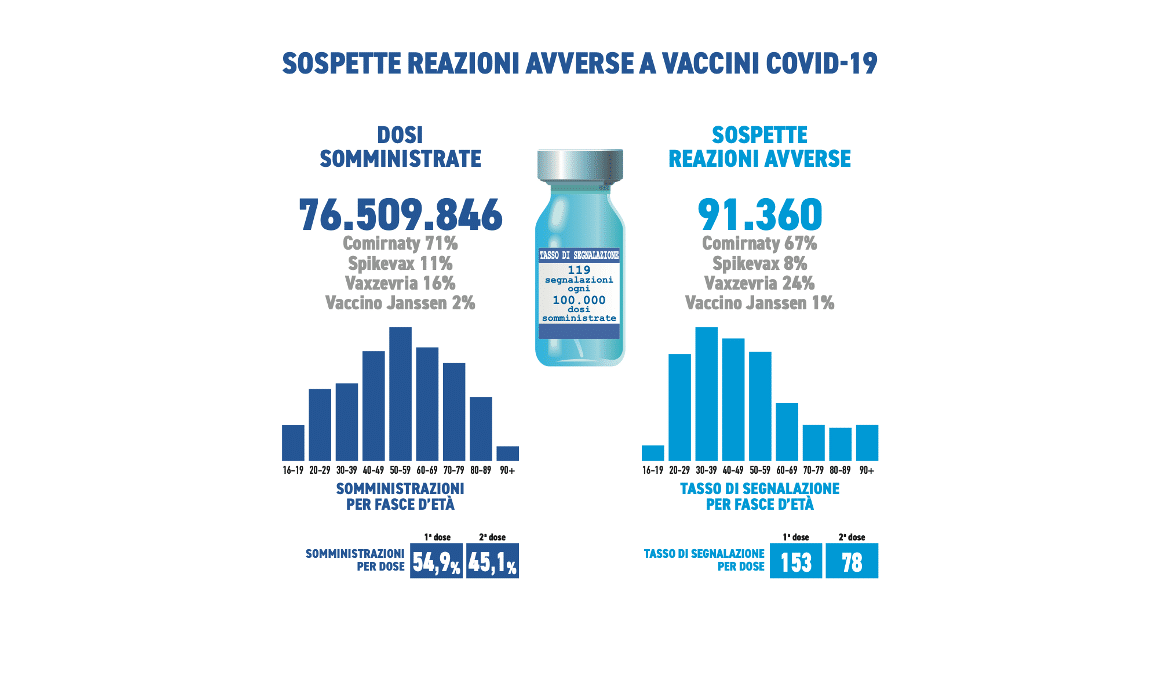
They were received during the period in question 91,360 reports out of a total of 76,509,846 doses administered (reporting rate of 119 every 100,000 doses), of which the86.1% referring to non-serious eventssuch as injection site pain, fever, asthenia/fatigue, muscle aches.
Serious reports correspond to 13.8% of the total, with a rate of 13 serious events per 100,000 administered doses. As reported in previous Reports, regardless of the vaccine, the dose and the type of event, the reaction occurred in most cases (approximately 80%) on the same day of the vaccination or the following day and only more rarely beyond the following 48 hours .
Comirnaty is the currently most used vaccine in the Italian vaccination campaign (71%), followed by Vaxzevria (16%), Spikevax (11%) and COVID-19 Janssen vaccine (2%). In line with previous Reports, the distribution of reports by type of vaccine follows that of administrations (Comirnaty 67%, Vaxzevria 24%, Spikevax 8%, COVID-19 vaccine Janssen 1%).

In relation to so-called heterologous vaccinations people under 60 who received Vaxzevria as the first dose were received 248 reports, out of a total of 604,865 administrations (the second dose concerned Comirnaty in 76% cases and Spikevax in 24% cases), with a reporting rate of 41 for every 100,000 doses administered.
In the age group between 12 and 19 years, as of 26/08/2021 have been received 838 reports of suspected adverse event out of a total of 3,798,938 administered doses, with a reporting rate of 22 adverse events per 100,000 administered doses. The distribution by type of adverse events is not substantially different from that observed for all other age groups.
Ottavo Rapporto (27/12/2020 – 26/08/2021)
Related news: Il direttore dell’Aifa Magrini: “I vaccini sono straordinariamente sicuri ed efficaci”