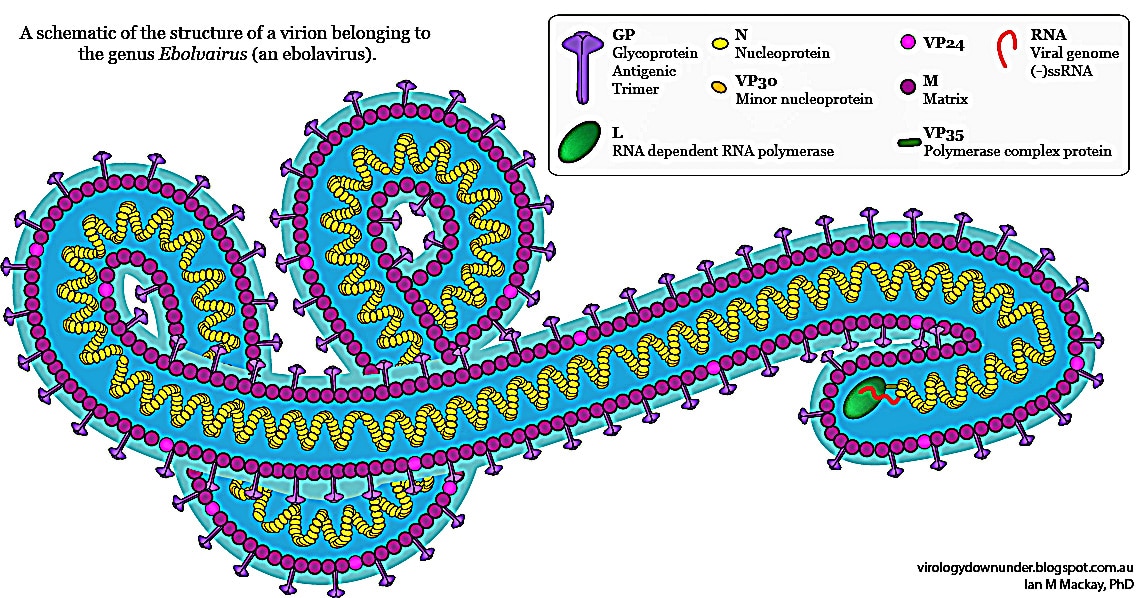
Falso allarme Ebola in Italia.Ebola. L’OMS valuta l’uso di farmaci sperimentali in Africa. Intanto il numero dei morti sale a 932
Verrà istituito un team di esperti che valuterà gli aspetti etici della vicenda. L’epidemia di ebola in Africa occidentale – riferisce l’Oms – ha contagiato già 1.711 persone, 932 di queste sono morte. Il ‘siero’ somministrato ai due missionari americani contagiati in Liberia non era mai stato usato prima con gli esseri umani, ma aveva dato buoni risultati sulle scimmie. Viene prodotto con la pianta del tabacco.
Geneva (Switzerland)
E’ sempre più emergenza ebola. I dati sono allarmanti: l’Organizzazione mondiale della Sanità ha riferito che i casi di contagio in Africa occidentale sono ormai già a quota 1.711 e 932 di questi hanno avuto come esito la morte. Dal 2 al 4 agosto, nei quattro Paesi interessati dall’epidemia – Guinea Conakry, Liberia, Sierra Leone e Nigeria – ci sono stati 108 casi nuovi e 45 decessi.
E anche il presidente degli Stati Uniti, Barack Obama ha riferito di non avere abbastanza informazioni sul nuovo farmaco ant-ebola: “Non ho abbastanza informazioni per considerare la procedura accelerata per l’approvazione”, ha detto. Ma è certo che il virus “si può controllare e contenere”. Il presidente ha però definito “prematuro” ricorrere all’uso di un farmaco sperimentale da
send to Africa to fight the virus and its spread. The leader of the White House then recalled that American investments in Africa amounted to 37 billion dollars.
Circa il nuovo farmaco, si parla del cosiddetto ‘siero miracoloso’, (vedi Zmapp, ed) quel farmaco sperimentale grazie al quale sarebbero migliorate le condizioni dei due missionari della Samaritan’s Purse Kent Brantley e Nancy Writebol ammalatisi in Liberia e ricoverati al centro sperimentale Usa di Atlanta per le cure. I due missionari, ora in isolamento, sono stati trattati con il ritrovato medico che avrebbe dato buoni risultati già a poche ore dalla somministrazione.
La Nigeria prende contatti con Atlanta per il ‘siero’
Anche il governo della Nigeria, uno dei paesi in ginocchio per l’epidemia, ha chiesto agli Stati Uniti l’acquisizione dello ZMapp. “Siamo in contatto con gli statunitensi. Martedì – ha detto il ministro della Salute – ho parlato per telefono con il direttore del Centro di Prevenzione e Controllo delle malattie di Atlanta, il dottor Tom Freiden. Abbiamo parlato a lungo e scambiato sms”, ha aggiunto il ministro.
Gli specialisti inglesi agli Usa: date il siero anche all’Africa
E l’appello agli Stati Uniti arriva anche dagli specialisti di Ebola del Regno Unito. Un appello affinchè il siero “miracoloso”, usato con i due americani rimpatriati lo scorso fine settimana, “venga dato a tutti nell’Africa occidentale”. Ora i tre specialisti, Peter Piot, che scoprì il temibile virus nel 1976, David Heymann, direttore del Chatham House Centre on Global Health Security, e Jeremy Trust del Wellcome Trust dicono che “ai governi africani dovrebbe essere consentito di prendere decisioni informate sull’usare o meno questi prodotti sperimentali”, riporta l’Independentt. Proprio i tre scienziati avevano chiesto all’Oms di attivarsi per fornire all’Africa occidentale le nuove medicine in fase di sperimentazione, anche considerando il propagarsi dell’epidemia. Anche da Liberia, Sierra Leone, Guinea, intanto, scrive ancora l’independent, calls for the liberalization of these substances are starting to arrive.
The controversy around the whey, produced with the tobacco plant
Lo ZMapp – ricordiamo – è un siero che non era mai stato usato prima con gli esseri umani e che aveva dato risultati promettenti nei test realizzati sulle scimmie. Crescono, quindi, le polemiche attorno al vaccino sperimentale. Il farmaco, non ancora approvato dalla Food and Drug administration, l’ente americano preposto al controllo di medicine e alimenti – è prodotto con la pianta del tabacco. E sembra funzionare. Per la realizzazione del vaccino, l’azienda farmaceutica Mapp Biopharmaceutical si avvale della collaborazione di Kentucky bioprocessing, filiale del gruppo americano del tabacco Reynolds American.
La prima a diffondere il nome del farmaco è stata l’emittente televisiva americana Cnn. Il siero è prodotto da Mapp, con sede a San Diego, in California. “Si è cominciato a ipotizzare l’utilizzo del tabacco per sviluppare il vaccino perché questa pianta è in grado di produrre rapidamente anticorpi in caso di emergenza”, ha detto Charles Arntezen, esperto di biotecnologia vegetale all’Arizona state university e che in passato ha collaborato con Larry Zeitlin e Kevin Whaley, i ricercatori che sono rispettivamente presidente e amministratore delegato di Mapp. Il processo di estrazione degli anticorpi “miracolosi” è lungo e complesso. Per produrre le proteine terapeutiche dentro la pianta del tabacco, i geni per gli anticorpi desiderati sono fusi assieme ai geni di un naturale virus del tabacco, ha aggiunto Arntezen. Le piante di tabacco sono poi infettate con questo nuovo virus artificiale. “La pianta, che a questo punto produce anticorpi per contrastare l’infezione – ha concluso – viene poi macinata e gli anticorpi vengono estratti”. Un processo, questo, che richiede settimane.
To find out more click: Marburg and Ebola Virus Infections by Craig R Pringle, BSc, PhD
Editorial: The disease and the interests of pharmaceuticals
Ebola: Il “fallimento morale” del settore farmaceutico
The Ebola hoax arrived in Italy
The news had bounced on Facebook launched by a far-right and xenophobic sympathizer craftsman. But Ebola has indeed arrived in Europe, albeit safely
(photo: Leon Neal/Afp/Getty Images)
"L'ebola she arrived at Italy, with three cases reported a Lampedusa. Fear of global epidemic”. The news, which appeared complete with accompanying photos of an immigrant marked by sores, had bounced on Facebook creating panic among readers. In a few hours it had reached 27,000 shares. But it was only of a hoax, soon unmasked by the investigations of the police post.
To throw the false alarm he was a 44-year-old craftsman from Turin, a far-right and xenophobic sympathizer, immediately tracked down and reported by the postal police for the dissemination of the photo and message, now removed from Facebook.
that theebola landed in Europe is however true. A 75-year-old Spanish missionary was in fact repatriated after contracting the virus in Liberia. Spain has in fact sent a military plane with a medical team on board to bring missionary Miguel Pajares home.
Meanwhile, after the administration of the experimental serum to the two Americans affected by the virus, also the WHO is consideringthe idea of employing drugs still being studied for the treatment of the African population affected by Ebola, where the dead are now close to a thousand. In fact, next week the WHO will convene a team of experts to evaluate, from a medical and ethical point of view, if, exceptionally and given the unusual circumstances, it is possible to imagine extendingAfrica the use of experimental treatments and who should benefit from it
Agnoletto and Aiuti: "Why the AIDS vaccine has failed"
The remote comparison on the anti-AIDS vaccine continues. After the blog with thespeech by Dr. Barbara Ensoli, head of the research project, here's what Aiuti and Agnoletto say, very critical of experimentation.
I thought that Ensoli had already responded sufficiently to the criticisms but no. In any case, here is what Aiuti and Agnoletto say, who largely repeat the things said, informally, so to speak, before the Senate Health Commission.
(The new installment of the blog-story on the Stsmina case will be released in the next few days).
Dear Pepper,
with this intervention, we intend to intervene on the scientific aspects of the anti-Tat vaccine and comment on some statements made by Dr. Barbara Ensoli, main responsible for the experimental vaccine in question, on her Blog and in other forums that can be consulted on the internet. The following are the essential points of our thinking.
1. Trial of the ISS therapeutic anti-Tat vaccine began in the experimental monkey model in 1998 and reached phase I (non-toxicity/safety study) in humans in 2003-5 and phase II (immunogenicity and determination of the optimal dose) in 2010, but has not yet provided results deemed of particular interest by the international scientific community after several million investments (at least 49, according to some sources; much less, but still many, according to Dr. Ensoli) and 16 years of research! In fact, there is no publication in the international literature - with the exception of the modest effects reported in the publications of Dr. Ensoli and previous works of the 90s by Daniel Zagury and Alessandro Gringeri - which has documented a clear efficacy in relation to the only two parameters considered essential for the control of HIV infection and its progression towards AIDS: (I) the levels of viral replication measurable in plasma (viremia) and (II) the percentage and/or absolute value of circulating CD4 T lymphocytes. On the basis of these parameters, from 1987 to today, all the current antiviral drugs on the market have been authorized in many countries of the world, drugs that so far are the only ones to have definitively blocked the progression of the disease in individuals and slowed down, at least in part, the global HIV pandemic.
2. The phase I experimentation of the ISS, both of the therapeutic and preventive vaccines, has raised criticisms at both a national and, above all, international scientific level, also due to the excessive and unjustified emphasis by the media. The same trial was also criticized by AIFA as evidenced by a document by 4 inspectors of AIFA itself who found 7 critical deviations and 3 major ones during the trial which took place in the period 2003-5. The journal Science on August 10, 2007 took up this debate and also criticized the direct funding of the experiment without there being a call for research open to various researchers. As regards the results of this painful phase I of the trial completed in 2005, we waited until 2009 for the publication of the results (Vaccine. 2009 May 26;27:3306-12.). But the publication, as underlined by Prof. Fernando Aiuti, does not report among the authors any doctor of the Policlinico Umberto I, Sapienza University of Rome who participated in the experimentation; obviously, none of them participated in the drafting of the work.
3. We also recall that on 11 June 2008 Maria Antonietta Coscioni, a member of parliament of the Democratic Party, presented a question to the Ministry of Labor and Social Policies and to the Ministry of Foreign Affairs (co-signatories Matteo Mecacci, Rita Bernardini, Elisabetta Zamparutti, Marco Beltrandi, all from the Democratic Party, and Giuseppe Giulietti from Italia dei Valori, joined by Donatella Poretti and Senator Marco Peduca) asking the ministers questioned "whether they do not deem it appropriate, in compliance with the principle of transparency and fairness, to respond to various points on the financing of the Tat vaccine". Other results of the therapeutic phase II were then published in 2010 in PlosOne (a generalist journal, considered to be of a different level than the most important journals) after an interim analysis and concerned patients from 11 Italian clinical centres. In our opinion, this work shows some critical issues as: a) it was conducted "open" (ie whoever administered the vaccine knew what he was administering) and not blinded; b) because the unvaccinated control group was selected on the historical basis of the archives of the various clinical centers and, lastly, c) because all the patients were on antiviral therapy with zero viraemia. In fact, the quantitative modifications of the main parameters in the vaccinated were insignificant.
4. It has also been 11 years since the initiation of phase I in humans of the preventive Tat vaccine; phase I ended in 2005, but in September 2011 the trial restarted again from phase I, and with a different clinical design which, in addition to the Tat protein, also included the Env protein supplied by Novartis. On 24 March 2014 – as can be verified on the ISS website – enrollment was suspended due to the non-compliance of Novartis' Env protein with the new European guidelines on quality documentation requirements relating to products used in clinical trials. In the meantime, international research has obviously continued producing other interesting vaccine candidates now in phases II and III. Among these, those conceived as plasmids containing viral DNA or the combination of at least three protein subunits of the HIV virus (Gag, Env, Nef or, possibly, Gag, Nef, Tat + Env). Therefore, there is no shortage of skills and lines of research pursued by the best international research centres. We wonder if it would not be appropriate to submit the results obtained from the experimentation of the ISS anti-Tat vaccine to a commission of international experts before deciding whether to continue the experimentation - with the obvious relative funding - in Italy as in South Africa.
5. The development of vaccines against other infectious diseases has never been blocked when the results have proved to be robust and sustainable only due to the fact that the pharmaceutical industries were afraid of losing a market for drugs directly or indirectly linked to these pathologies. In this regard, Dr. Ensoli declares "... who will give me 100-150 million € to conclude the trial?" Today the cost of developing a new drug from its discovery to its commercialization is estimated at between €250 and €400 million, costs that large industries have no problems with. Thus, the ISS package of patents relating to the anti-Tat vaccine could be sold or licensed for use by specialized industry at a profit for the public institute (as well as for the inventors of the patents). Obviously, the industrial partner must "believe" in the potential goodness of the drug (a therapeutic vaccine can be considered as such) or of the preventive vaccine in question in order to invest in it, an event which, to date, has not occurred for the anti-Tat vaccine of the ISS.
6. On this issue, it is also worth recalling the story that gave rise to a recent national debate. In March, the Board of Directors of the ISS (Resolution No. 7 of 2014) granted the license for the TAT vaccine for 18 months to a private company, Vaxxit Srl with a share capital of €10,000 and with the 70% of ownership shares held by Dr. Ensoli herself, for the 30% to a former ISS consultant (Dr. Giovanni Battista Cozzone, patent expert). After the public criticisms, raised in particular by the monthly Altreconomia received by the operation and the parliamentary question of Sen. Emilia De Blasi, President of the Senate Health Commission, in June the ISS Board of Directors took a step back by withdrawing ( at least temporarily) the resolution granting Vaxxit Srl. In the meantime, the ISS, for the first time in its history, was commissioned and the resolution is now in the hands of the new ISS commissioner. In the meantime, new shareholders have entered Vaxxit Srl – which appears to have started looking for capital on the market – including Dr. Ensoli's brother, Dr. Fabrizio Ensoli of San Gallicano (another recently commissioned institution), his ex-husband, Dr. Aurelio Cafaro, researcher at ISS, the largest Canadian fund (Teralys Capital) and a credit multinational (Ferghana Securities Inc.) domiciled in Delaware, USA, an American state with reduced taxation, as well as a representative of an Italian NGO that has long stood out for having taken sides in public defense of the ISS anti-Tat vaccine.
You see, dear Pepe, if Dr. Ensoli's vaccine study had once returned to the scope of the AIDS Project, whose funding was the result of healthy competition between the best projects thanks to the "peer review" mechanism, it would not have had probably nothing outrageous. Instead this project was financed for tens of millions of euros directly by government decisions without prior consultations with independent national and international experts. It should be added that over the years the results have been modest at best, without any relevant efficacy on viraemia and values of CD4 lymphocytes too spread over time and with uncertain development prospects, as demonstrated by the fact that the main pharmaceutical companies specializing in the development of vaccines have not come forward to take over or have the ISS patents licensed for use.
We hope that the recent hearing in the Senate (mentioned above) at the Health and Hygiene Commission of some experts involved in this matter will bring useful suggestions to the current Government, to the new Commissioner of the ISS and to the Ministers of Health and Foreign Affairs.
We, the signatories of this document, have different political opinions, but in this case we share the idea that quality research and the promotion of prevention should be financed in Italy through an evaluation system that involves both national and international experts to formulate an informed choice of the most current and important issues through a competitive and transparent tender without conditioning or bypassing politics or preferential lanes as occurred in the case of the ISS vaccine candidate based on the viral protein Tat. The problem is urgent because the HIV epidemic in Italy continues to infect about 4,000 people every year and about 50% of individuals discover they are infected only when they are already in the full-blown AIDS phase. Giving up public research free from conditioning by the political world and the pharmaceutical industry means giving up a little freedom and abdicating the status of a civilized and advanced country such as Italy still aspires to be.
Cordially,
Prof. Vittorio Agnoletto
State University, Milan
Prof. Fernando Aiuti
Professor Emeritus University "Sapienza" Rome
(the views expressed here are personal and are not intended to represent in any way the opinion of the institutions in which the signatories work or have worked in the past years)
guglielpepe@gmail.com
@pepe_guglielmo (Twitter)