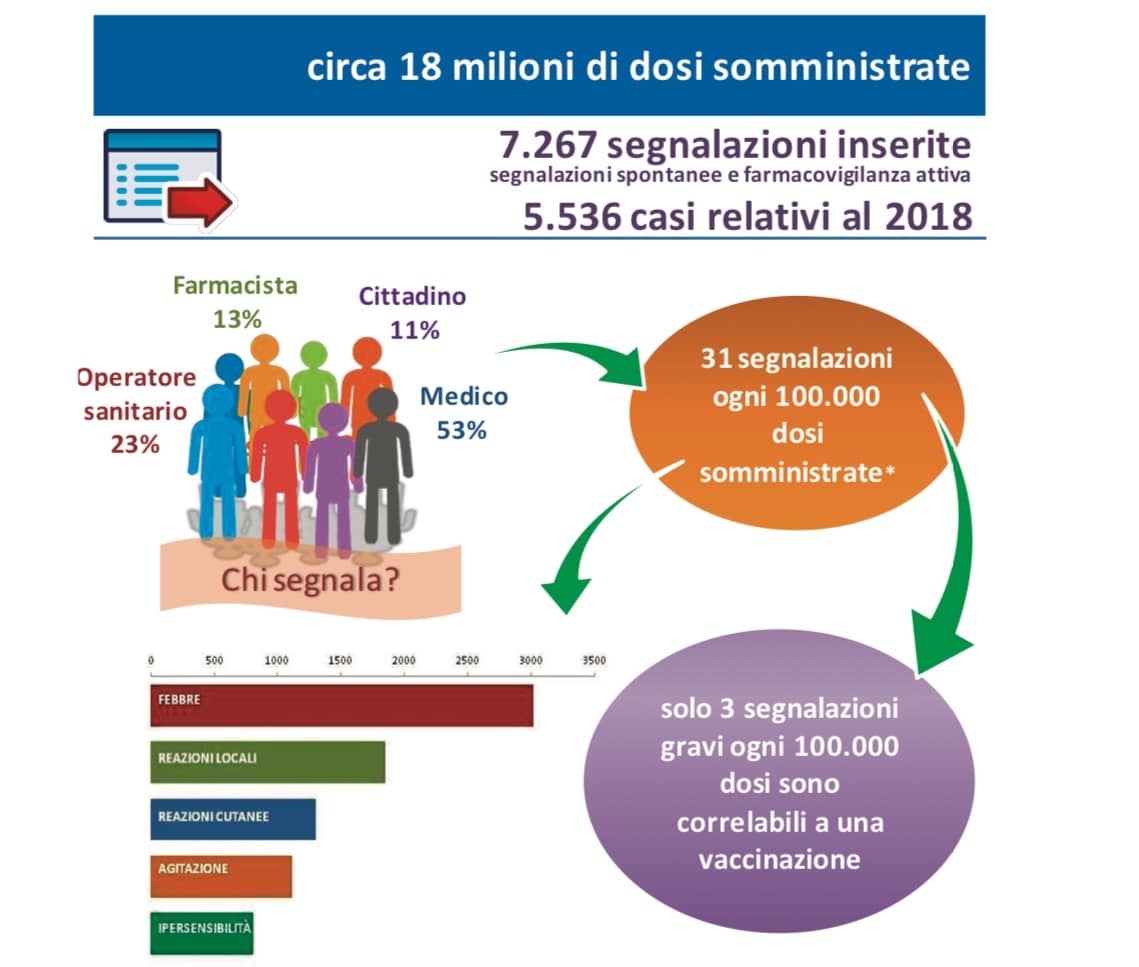
The Vaccine Report summarizes the post-marketing surveillance activities on vaccines conducted in Italy in 2018. Compared to previous reports, this year it was possible to use, for the calculation of the reporting rates (ratio between the number of reports and the data of exposure), the doses actually administered at national level, provided by the Ministry of Health and by the Departments of Prevention of the Regions and Autonomous Provinces.
The reporting frequency of correlated serious adverse reactions is 3 events per 100,000 doses. The correlated reactions reported are all known and therefore already reported in the product information of the vaccines authorized in Italy.
L’andamento crescente del numero delle sospette reazioni avverse è indicativo di una sempre maggiore attenzione alla vaccinovigilanza da parte sia degli operatori sanitari che dei cittadini. Dall’analisi dei dati nazionali, non sono emerse informazioni che possano influenzare il rapporto beneficio-rischio per le varie tipologie di vaccino correntemente utilizzate, confermando quindi la loro sicurezza.
In addition to the in-depth analysis of suspected adverse reactions for each type of vaccine, as every year, a focus was dedicated to anti-flu vaccines and some insights which, in this edition, concern international vaccinations for travelers and the vaccinations recommended in pregnancy.
AIFA Published on: 30 July 2019