The Italian Medicines Agency (Aifa) published the «Monitoring of national and regional pharmaceutical expenditure» referring to the period January-September 2022. Pharmaceutical expenditure, calculated at 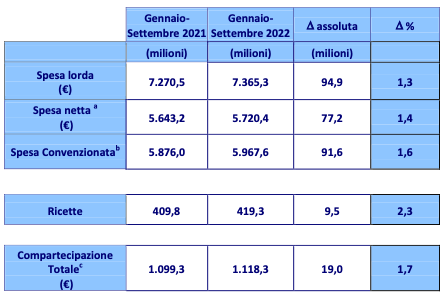
This highlights a slight increase, compared to the previous year, of +77.2 million euro. The number of prescriptions disbursed (419.3 million) showed the same trend with respect to this increase, showing a change of +2.3% on 2021. The incidence of the ticket, i.e. the "out of pocket" expense of patients, increased by +1.7%. As for drug consumption, the number of daily doses shows an increase of +0.5%, equal to 87.8 million dispensed.
With reference to the regional approved pharmaceutical expenditure incurred through the payment of the summary accounting slip to private and public territorial pharmacies, the Aifa report shows that the Regions with the greatest percentage increase are Lombardy (+4.6%), PA Trento (+4 ,2%) and Molise (+3,8%).
The regions with a decrease are Umbria (-4.0%), Sicily and Campania (-1.1%). In most of the Regions there is an increasing trend of the concessions, with a recovery compared to the negative trend highlighted in the previous year. Other regions with a positive gap include Abruzzo (+3.0%) and Emilia-Romagna (+2.6%).
The monitoring of pharmaceutical expenditure that AIFA periodically publishes is an essential prerequisite for the planning of pharmaceutical assistance in Italy.
L’AIFA effettua il monitoraggio mensile dei dati di spesa farmaceutica e comunica le relative risultanze al Ministero della salute e al Ministero dell’economia e delle finanze con la medesima cadenza. Al 31 maggio, al 30 ottobre e al 31 dicembre di ogni anno l’Agenzia verifica l’eventuale superamento a livello nazionale dei tetti di spesa, sia della spesa farmaceutica convenzionata (7,96% del fabbisogno sanitario nazionale) sia per acquisti diretti (6,69% + 0,2% del fabbisogno sanitario nazionale).
The monitoring of pharmaceutical expenditure is conducted on the basis of the agreed expenditure data and the Summary Accounting Lists (DCR) acquired by the Regions, as well as the data acquired by the New National Information System (SIS) of the Ministry of Health, relating to the traceability of the drug (DM July 15, 2004).
A decorrere dal 1° gennaio 2017, nello stato di previsione del Ministero della Salute, è stato istituito un Fondo per il concorso al rimborso alle Regioni per l’acquisto dei medicinali innovativi non oncologici, con una dotazione di 500 milioni di euro annui e un Fondo per il concorso al rimborso alle Regioni per l’acquisto dei medicinali oncologici innovativi, con una dotazione di 500 milioni di euro annui.
