Sanofi and GSK COVID-19 vaccine, confirmation of strong immune response from phase II. We move on to phase III
 Sanofi and GSK's adjuvanted recombinant COVID-19 vaccine candidate achieved strong rates of neutralizing antibody responses, consistent with those measured in people who have recovered from COVID-19, across all adult age groups in a Phase 2 study with 722 volunteers.
Sanofi and GSK's adjuvanted recombinant COVID-19 vaccine candidate achieved strong rates of neutralizing antibody responses, consistent with those measured in people who have recovered from COVID-19, across all adult age groups in a Phase 2 study with 722 volunteers.
A global Phase 3 study is expected to begin in the coming weeks and, if yielding the expected results, following necessary regulatory reviews, should be approved in the fourth quarter of 2021.
The candidate adjuvanted recombinant COVID-19 vaccine by Sanofi and GSK achieved strong rates of neutralizing antibody responses, consistent with those measured in people who have recovered from COVID-19, across all adult age groups in a Phase 2 study of 722 volunteers.
THE Phase 2 interim results showed seroconversion from 95% to 100% after a second injection in all age groups (18 to 95 years) and in all doses, with acceptable tolerability and no safety concerns. Overall, the vaccine candidate elicited strong levels of neutralizing antibodies that were comparable to those generated by natural infection, with higher levels seen in younger adults (18 to 59 years). After a single injection, high levels of neutralizing antibodies were generated in participants with evidence of prior SARS-CoV-2 infection, suggesting strong development potential as a booster vaccine.
 “Our Phase 2 data confirms the potential for this vaccine to play a role in addressing this ongoing global public health crisis, as we know more vaccines will be needed, especially as variants continue to emerge and the need for effective vaccines and recall, which can be stored at normal elevated temperatures, increases,” he said Thomas Triomphe, Executive Vice President and Head of Sanofi Pasteur. “With these favorable results, we are ready to move into a global Phase 3 efficacy study. We look forward to generating additional data and working with our partners around the world to make our vaccine available as quickly as possible.” possible ".
“Our Phase 2 data confirms the potential for this vaccine to play a role in addressing this ongoing global public health crisis, as we know more vaccines will be needed, especially as variants continue to emerge and the need for effective vaccines and recall, which can be stored at normal elevated temperatures, increases,” he said Thomas Triomphe, Executive Vice President and Head of Sanofi Pasteur. “With these favorable results, we are ready to move into a global Phase 3 efficacy study. We look forward to generating additional data and working with our partners around the world to make our vaccine available as quickly as possible.” possible ".
Roger Connor, president of GSK Vaccines added: “These positive data show the potential of this protein-based adjuvanted vaccine candidate in the broader context of the pandemic, including the need to address variants and anticipate booster doses. We believe this vaccine candidate can make a significant contribution to the ongoing fight against COVID-19 and will move into Phase 3 as soon as possible to meet our goal of having it available later this year.”
Based on these positive interim Phase 2 results, the companies plan to initiate a global, randomized, double-blind Phase 3 study with the 10 µg dose, in combination with GSK's pandemic adjuvant, in the coming weeks. The Phase 3 study is expected to enroll more than 35,000 adult participants from a broad range of countries and evaluate the efficacy of two vaccine formulations, including D614 variants (Wuhan) And B.1.351 (South African).
___________________________________
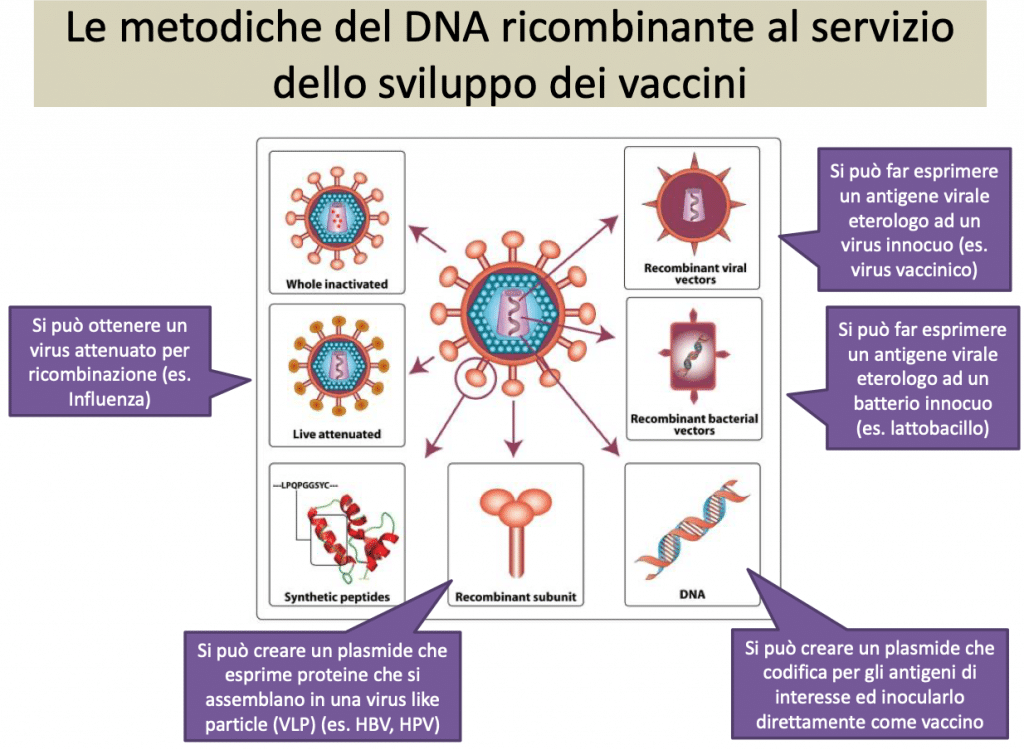 Note: The vaccine candidate, co-developed by Sanofi and GSK, is a subunit recombinant DNA vaccine based on an established technology and is the same one that Sanofi already successfully adopts for the production of the recombinant quadrivalent influenza vaccine. The adoption of this technology will make it possible to produce a significantly greater number of doses. The Phase 1/2 clinical trial is a randomized trial. (Sanofi source). GSK supplies the adjuvant.
Note: The vaccine candidate, co-developed by Sanofi and GSK, is a subunit recombinant DNA vaccine based on an established technology and is the same one that Sanofi already successfully adopts for the production of the recombinant quadrivalent influenza vaccine. The adoption of this technology will make it possible to produce a significantly greater number of doses. The Phase 1/2 clinical trial is a randomized trial. (Sanofi source). GSK supplies the adjuvant.
In short, vaccinating with DNA consists in transmitting to the host cells the genes that contain the information necessary for the cell to produce antigens of the SARS-CoV-2 "spike" protein, the protein that will provoke a specific immune response with direct antibodies against the same spike protein.
The genes are inserted in the laboratory into a virus that is harmless to humans (the so-called vector) which, once injected, carries the genes up to the cell. Here the injected genes behave like any other gene: they are copied into messenger RNA (mRNA) molecules that reach the ribosomes - the cell's microscopic protein factories - and make them produce the "spike" protein.
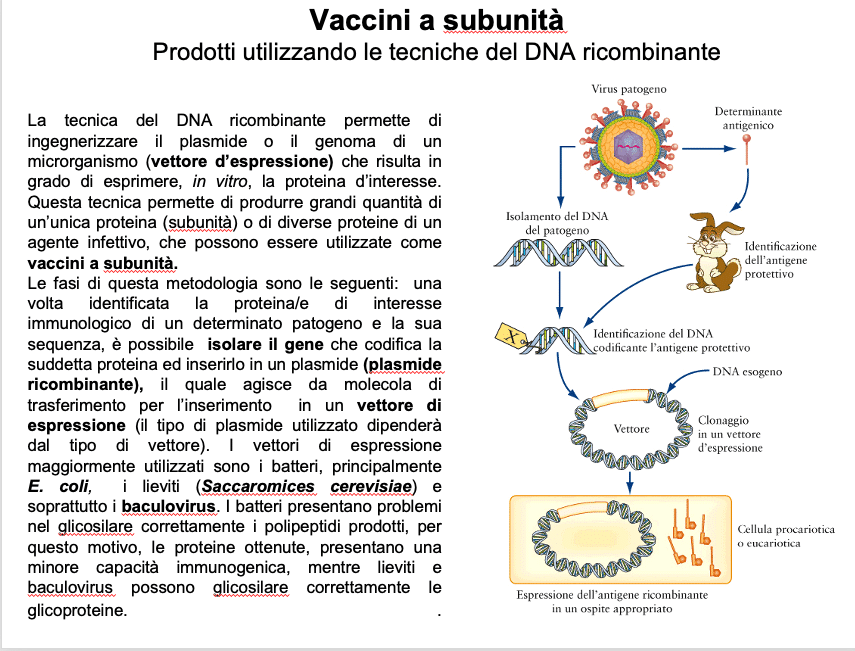 Recombinant DNA is used to produce GMOs capable, for example, of functioning as bioreactors for the production of hormones for medical use, such as insulin, growth hormone or oxytocin, or vaccines. The most used bioreactor, also because it is the easiest to handle, is Escherichia coli, a prokaryotic bacterium in which a gene can be inserted to be translated abundantly into protein (as occurs in the cases just reported). Given the poor immunogenicity of peptide vaccines and the poor efficacy of sub-unit vaccines in inducing a cell-mediated response, new adjuvant technologies have been developed for the enhancement of peptide or sub-unit vaccines.
Recombinant DNA is used to produce GMOs capable, for example, of functioning as bioreactors for the production of hormones for medical use, such as insulin, growth hormone or oxytocin, or vaccines. The most used bioreactor, also because it is the easiest to handle, is Escherichia coli, a prokaryotic bacterium in which a gene can be inserted to be translated abundantly into protein (as occurs in the cases just reported). Given the poor immunogenicity of peptide vaccines and the poor efficacy of sub-unit vaccines in inducing a cell-mediated response, new adjuvant technologies have been developed for the enhancement of peptide or sub-unit vaccines.
Sanofi is also co-developing an mRNA vaccine for COVID-19 with Translate Bio, which is on pace to begin human trials this quarter. Meanwhile, GSK and Vir Biotechnology launched phase III trials last year for VIR-7831, a monoclonal antibody therapy for adult patients hospitalized for COVID-19.
Related news: Ministry of Health. Anti-Covid 19 vaccines