AIFA
-
News
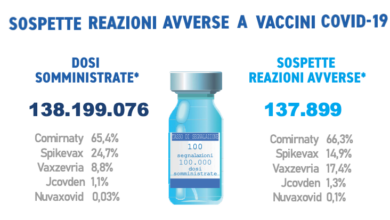
AIFA. The XIIth report on the surveillance of anti-COVID-19 vaccines has been published
L’Agenzia Italiana del Farmaco ha pubblicato il dodicesimo Rapporto di farmacovigilanza sui vaccini anti-COVID-19. I dati raccolti e analizzati riguardano…
Leggi » -
First floor

AIFA. Presented the OsMed report on the use of drugs in Italy. The case of vitamin D
È stato presentato il 29 luglio il Rapporto Nazionale 2021 “L’uso dei Farmaci in Italia”, realizzato dall’Osservatorio Nazionale sull’impiego dei…
Leggi » -
News

EMA. Towards better prevention of medicine shortages in the EU
L’EMA ha pubblicato una guida per le organizzazioni di pazienti e operatori sanitari con principi chiave ed esempi di buone…
Leggi » -
News

EMA. Vaccine safety evaluation
L’Agenzia europea per i medicinali (EMA) monitora con estrema attenzione la sicurezza dei vaccini COVID-19 autorizzati nell’Unione europea (UE). Ciò…
Leggi » -
News

AIFA. The 5% fund is under management: it is intended for the use of orphan drugs for the treatment of rare diseases
New online service for the management of the AIFA 5% Fund From 1 July 2022, access to the AIFA 5% National Fund…
Leggi » -
News

Critical drugs in Europe. EMA activates i-SPOC, single point of contact for the sector which will inform EMA
I titolari dell’autorizzazione all’immissione in commercio (AIC) possono ora registrare il loro , (i-SPOC) della fornitura e della disponibilità…
Leggi » -
News

The purchase of drugs in the Cnn class: critical issues and addresses at a legal level
In 2012, with the law n. 189, also known as the Balduzzi Decree, the legislator intended to provide a new instrument…
Leggi » -
News

AIFA. Launch of the "TrovaNormeFarmaco" portal
L’Agenzia Italiana del Farmaco ha attivato il portale TrovaNormeFarmaco con l’obiettivo di favorire la ricerca e la consultazione della normativa…
Leggi » -
First floor

Prof. Garattini: "True independent information on medicines is urgently needed". Ed
On Thursday 16 June, L'Avvenire published an Appeal by Prof. Silvio Garattini entitled "True independent information on…
Leggi » -
News

New reports of adverse reactions to drugs and vaccines
With the launch of the new National Pharmacovigilance Network (RNF), the new forms will come into force on 20 June 2022…
Leggi »
