
Participating in a clinical trial offers significant benefits. What is missing, however, is the awareness of this opportunity. Patients and caregivers increasingly protagonists
Veronesi Foundation – 14 December 2021
If there's one thing we've touched during this pandemic, it's the little knowledge by the Italians in the context of the clinical trials of drugs. The adjective "experimental" is increasingly assuming a negative meaning even if, in reality, it is thanks to clinical trials that life expectancy has lengthened, if new treatments have been discovered and if, ultimately, medicine progresses. This reticence towards experimentation has to do with a lack of culture and, let's add, even a bit of stigma. From us who participates in one clinical study he is seen as a "Big Pharma guinea pig" and instead the numerous advantages, for himself and for the system, of experimentation are not grasped. Because, in essence, of clinical trials you have confused ideas. In the last ten years, at European and Italian level, various realities have arisen that promote knowledge of scientific research among citizens and patient associations are showing themselves to be increasingly active and eager to participate in the research. But there is still a long way to go.
 TO RAISE AWARENESS
TO RAISE AWARENESS
And not only the patients, but also those who govern us. The Stamina case it will remain in the history of our country not only as one of the greatest scientific fraud of recent times, but also for how it was managed at the parliamentary level, despite the opposition of the entire scientific community: «With the Stamina case I understood that this country needed help, because the parliament and those who govern us cannot fail to know - he recalls Dominique Van Doorne, endocrinologist and president of the EUPATI Expert Patient Academy (ADPEE)– and the pandemic has convinced me even more that the citizens of experimentation and research know very little about it. Let's take the example of vaccines antiCOVID: even if we have high rates of vaccination does not mean that this is due to a full understanding of science. Many people have been vaccinated even if they are undecided or fearful ».
And this despite sui vaccines has been activated the most extensive pharmacovigilance of history: we know more about this vaccine than about others that have already been known for some time, but on which surveillance of this magnitude has never been activated. «That's why it's important to know how clinical trials work – continues Van Doorne – because one would know that the first thing that is tested is the safety of the drug, even before its effectiveness. Before moving on to the in vitro phase, the computer is used and the various interactions of the molecules are analyzed at a computational level. Then we move on to the in vitro analysis, then on the animal one and finally the clinical one (on humans) ». Knowing how experimentation works helps acUnderstand how search works, medicine and, ultimately, how medicines are made. Knowing is the first step in eliminating doubts and fears and in becoming increasingly aware of one's own well-being. This is why various realities have arisen, such as EUPATI and ECRAN, which aim at the empowerment of the patient and the caregiver, increasingly important interlocutors in the field of clinical trials.
THE EUPATI AND ECRAN PROJECT
The European Patients' Academy on Therapeutic Innovation (EUPATI) was founded in 2012 on the initiative of the Innovative Medicines Initiative (IMI), a public-private partnership between the European Commission and EFPIA (the association of pharmaceutical industries in Europe) which has the to develop new treatments and treatments. EUPATI's mission is to give citizens a better understanding of how medicines development works and how they can be actively involved in the process. Becoming experienced patients and caregivers, these subjects can interact in an authoritative way with regulatory authorities, research representatives and institutions. EUPATI arrives in Italy with the EUPATI Expert Patient Academy, founded in 2014 in Rome which every year since 2018 organizes an annual course, with free access, aimed at patients and caregivers and created with the support of AIFA, the patronage of the Institute Superiore di Sanità and with the non-conditioning contribution of pharmaceutical companies. In 2014 the ECRAN project was born - European Communication on Research Awareness Needs - funded by the European Community to improve the knowledge of European citizens on medical research and support their participation in independent clinical trials. ECRAN intends to provide clear and reliable explanations of basic and advanced concepts, practical information, educational content such as guides, tutorials, films and even games to help citizens make an informed choice when deciding to participate in a clinical study. The project coordinator is the Mario Negri Institute in Milan. ECRAN is currently paused, but the site is still accessible.
 THE ADVANTAGES OF CLINICAL TRIAL
THE ADVANTAGES OF CLINICAL TRIAL
Understand how the experiment works clinic removes stigma, fear, reticence and allows you to understand their advantages. One above all: the possibility of being able to access innovative treatments. For those suffering from rare diseases, for example, participating in clinical trials can be a way to access otherwise inaccessible treatments. For women affected by ovarian cancer, another example, joining trials represents the only possibility of participating in the improvement of treatments. But although more than half of the patients are in favor, only one in ten women are offered to participate. He revealed it Research inActo, a study promoted by the patient association Alliance against the ovarian cancerACTO-ONLUS and conducted by the Mario Negri IRCCS Institute of Milan which involved 359 patients. About six out of ten women know what a clinical study is, but only 34.9% knows how it is carried out. And it is almost always the doctor who proposes to participate, and he does so only in 12% of cases. In this sense, patient associations are assuming an increasingly decisive role in providing support to facilitate participation in trials. The other advantages of experimentation concern the possibility of be thoroughly examined and this brings benefits not only for patients (who can receive a complete check-up that otherwise would have taken months to obtain) but also for the national health system, given that the costs of the trial are borne by those who sponsor them (in most of cases, pharmaceutical companies) and a thoroughly controlled person, thanks to the clinical study, will certainly turn less to the NHS.
THE ROLE OF SCIENTIFIC SOCIETIES
Patient organizations can do a lot, but not everything. In this path of continuing education of patients and caregivers, a decisive role must also be played by scientific societies. Some of these, such as the FADOI (Federation of Associations of Hospital Internist Managers) have collaborated with ADPEE, to produce an important document entitled "Inside Research: the Person first of all - A proposal in 10 points", signed by over 200 Scientific Associations, IRCCS, Ethics Committees, Citizens and Patients Associations and intended for the world of research and institutions to propose ten recommendations 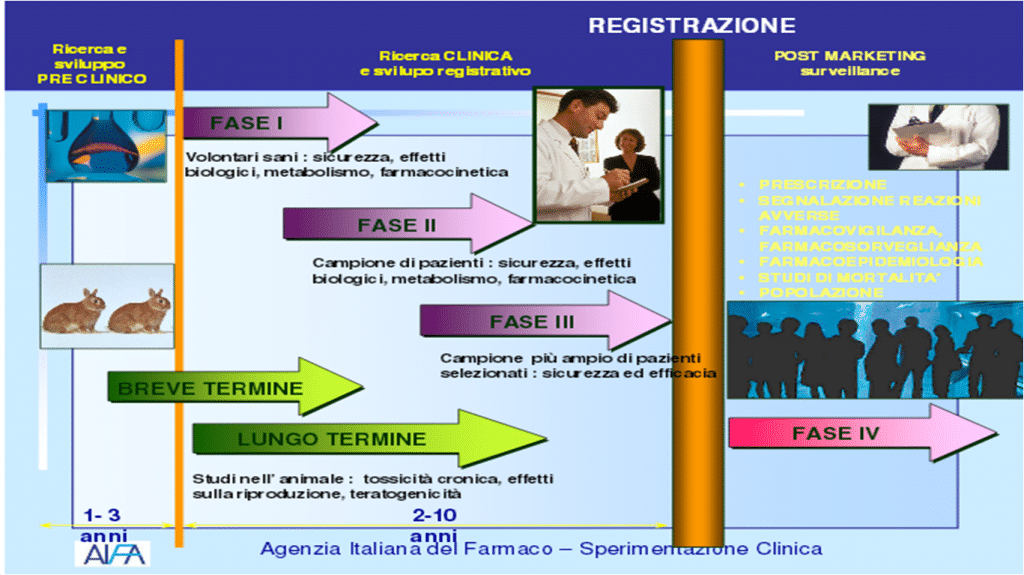 pragmatics on clinical trials in Italy. But in addition to the recommendations, there is a need for those who do research to start actively collaborate with patients, through the use of simple language and the involvement of patients in the very definition of the hypotheses on which the studies are based: «With the pandemic we are realizing how much there is a need for aware patients – explained Gualberto Gussoni, FADOI scientific director – and we as researchers and doctors must cement the relationship of trust between those who propose the research and those who must participate in it. Today the patient associations they are much more active and protagonists. They work hard to understand better, while we should work harder to make ourselves better understood. And instead we're in our ivory tower, we don't have a real perception of reality. We should work more in terms of dissemination, explain why research is carried out and what needs it responds to».
pragmatics on clinical trials in Italy. But in addition to the recommendations, there is a need for those who do research to start actively collaborate with patients, through the use of simple language and the involvement of patients in the very definition of the hypotheses on which the studies are based: «With the pandemic we are realizing how much there is a need for aware patients – explained Gualberto Gussoni, FADOI scientific director – and we as researchers and doctors must cement the relationship of trust between those who propose the research and those who must participate in it. Today the patient associations they are much more active and protagonists. They work hard to understand better, while we should work harder to make ourselves better understood. And instead we're in our ivory tower, we don't have a real perception of reality. We should work more in terms of dissemination, explain why research is carried out and what needs it responds to».
«The topic is important and we must take action, as EUPATI did - concluded Gussoni - also because the patient must be an active part of the trial design: we researchers often activate studies on the basis of academic hypotheses, not looking at the real needs of patients . Instead, we should consider patient reported outcomes more, as well as the logistical needs of patients: tomorrow's trials will be increasingly organized at the patient's home, through tools such as telemedicine". But to obtain an active participation of the population, it is first necessary to train them on the importance of clinical trials.
SOURCES
Related news: AIFA. Clinical trials of drugs
The documents reported represent opinions, interpretations and reconstructions, even subjective ones and not necessarily shared by the editorial staff. Therefore, the responsibility of the statements lies with the author and/or whoever has provided us with the content. Our intention is to provide useful information for scientific representatives and to disseminate news of public interest. Naturally we invite readers to always deepen the subject matter, to consult more sources and we leave each of them the freedom of interpretation. The material found on the net, where the copyright wording and the rights of use have not already been declared on the site, has been deemed to be in the public domain in good faith. Some texts quoted or images included in this site are taken from the internet and, therefore, considered to be in the public domain; if their publication violates any copyright, please notify us via e-mail to provide for the consequent removal or modification.





