The need to access structural information on product protection certificates emerged from the technical table on pharmaceutical governance and medical devices set up in early August
– August 27, 2018 – AboutPharma
On the subject of the patent expiry there is an agreement between Mise and the Ministry of Health. The purpose of the request made by Giulia Grillo's dicastery was to facilitate access and consultation of the patent databases (which contain deadlines and certificates of complementary protection).
Speed up processes
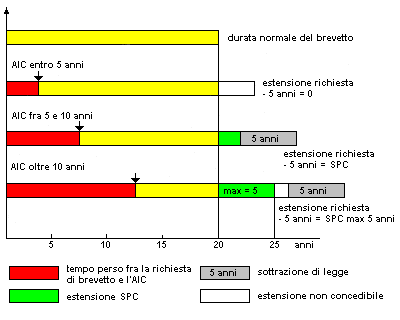 The request arises from the need of the technical offices of AIFA and the Regions to be able to access this precious data in an organic and timely manner. This pressing need emerged at the Technical table on pharmaceutical governance and medical devices recently set up at the Ministry of Health. According to experts, accelerated processes are needed to be able to deal with a periodic update of the national pharmaceutical formulary. In fact, equivalent medicines, as known, cannot be classified by the National Health Service with effect prior to the expiry date of the patent or the complementary protection certificate.
The request arises from the need of the technical offices of AIFA and the Regions to be able to access this precious data in an organic and timely manner. This pressing need emerged at the Technical table on pharmaceutical governance and medical devices recently set up at the Ministry of Health. According to experts, accelerated processes are needed to be able to deal with a periodic update of the national pharmaceutical formulary. In fact, equivalent medicines, as known, cannot be classified by the National Health Service with effect prior to the expiry date of the patent or the complementary protection certificate.
Fast times
"It is quite evident that the possibility of finally being able to acquire the data in question quickly - wrote the Minister of Health, Giulia Grillo, to the Minister of Economic Development, Luigi Di Maio - assumes importance and a strategic impact for the purposes of classification of medicines by the National Health Service. For this, I thank the Mise for having filled a historic information gap, allowing us at this point to proceed with greater certainty and with a precise picture of the situation".
Related news: Community and national rules on generics