COVID-19. China has patented its first coronavirus vaccine
China has given its first approval to a possible vaccine against Covid-19: is the Ad5-nCoV,  developed by CanSino Biologics, in collaboration with the Biotechnology Institute of the Academy of Military Medical Sciences. The Chinese pharmaceutical company according to Chinese state media in March had been the first to bring a vaccine to the experimental phase.
developed by CanSino Biologics, in collaboration with the Biotechnology Institute of the Academy of Military Medical Sciences. The Chinese pharmaceutical company according to Chinese state media in March had been the first to bring a vaccine to the experimental phase.
As last week's news about the vaccine developed and registered in Russia, even today's one should be taken with great caution. According to official data from the World Health Organization, neither the vaccine registered in Russia nor the one patented by CanSino is among the six that are currently in "phase 3" of experimentation, i.e. the most advanced and reliable for evaluating its efficacy, but even the most delicate.
According to Chinese state media, the "phase 3" trial of the patented vaccine is expected to begin this month in Saudi Arabia, while CanSino said it was in talks with other countries - Russia, Brazil and Chile - to start further trials.
Meanwhile CanSino Biologics shares, following the latest developments, posted solid gains on the Hong Kong (+13.90%) and Shanghai (+4.33%) stock exchanges.
Related news:
Incorporated in 2009, CanSinoBIO (SHSE: 688185, HKEX:06185) commits to research, production and commercialization of innovative vaccines for China and global public health security. It possesses four integrated platform technologies including adenovirus-based vectors, conjugation, protein design and recombination and formulation. As of today, it has established a robust pipeline of 16 vaccines preventing 13 diseases, including a globally innovative Ebola virus vaccine approved in 2017 as well as the investigational Recombinant Novel Coronavirus Vaccine (Adenovirus Type 5 Vector). Additional information can be found online at www.cansinotech.com.
RESEARCH INFO
The research involved 320 "healthy volunteers" between the ages of 18 and 59, 96 of whom participated in Phase 1 and 224 in Phase 2. According to Xinhua, the results indicate that the vaccine actually induced neutralizing antibodies in the volunteers and demonstrated a "good ability to procure an immune response".
WHERE THE STUDIES WERE CONDUCTED
The studies were conducted by the Wuhan Institute of Biological Products and the Wuhan Institute of Virology under the auspices of the Sinopharm-affiliated National Biotechnology Group of China (CNBG) and the China Academy of Sciences, he wrote. Nova Agency.
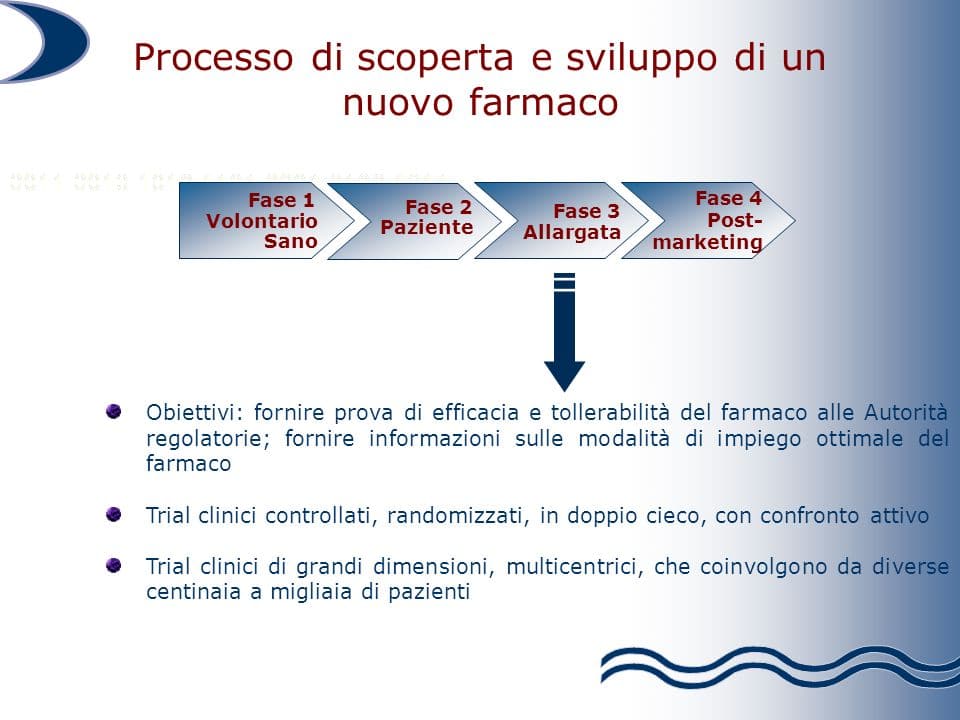
Phase 2 is useful in demonstrating the non-toxicity and activity of the new one active principle experimental.
However, there are still other questions that need to be answered: how effective is the drug? Does it have any additional benefits compared to similar drugs already on the market? And what is the relationship between risk and benefit?
Phase 3
All these questions are answered with the study of stage 3 (or therapeutic-confirmatory). In this case, the "enlisted" patients are no longer a few dozen, but hundreds or thousands. The drug's efficacy on symptoms, quality of life, or survival is compared to a placebo (substance without therapeutic efficacy), with other drugs already in use, or with no treatment.
Related news: Putin surprisingly announces anti-Covid vaccine. But phase 3 seems to be missing. Perplexity from the West





