Il 21 ottobre scorso si è tenuto presso la sede della Regione Emilia Romagna l’incontro del Gruppo di lavoro sull’Informazione scientifica nell’ambito del SSR coordinato dalla Dr.ssa Valentina Solfrini, Dirigente di riferimento Area Farmaci e Dispositivi Medici della Direzione Generale Cura della Persona, Salute e Welfare dell’Assessorato Politiche della salute della Regione Emilia Romagna.
Thanks to the Fedaiisf contribution, the system will be improved and brought closer to working reality.
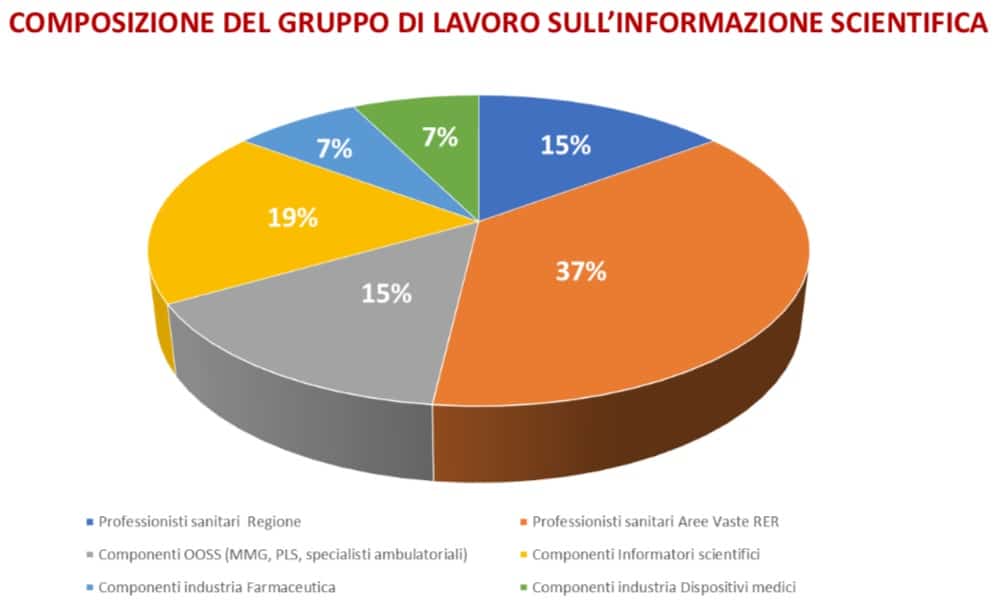
Fedaiisf was present in the working group representing the Pharmaceutical Representatives (ISF), whose code of ethics is recognized by the Region as a value. ISF was also present for the trade union representatives.
During the meeting, a single regional application was thought of which provides for the insertion of personal data, of the subject matter, of the professional/s that one/they want to meet. It is also expected to happen 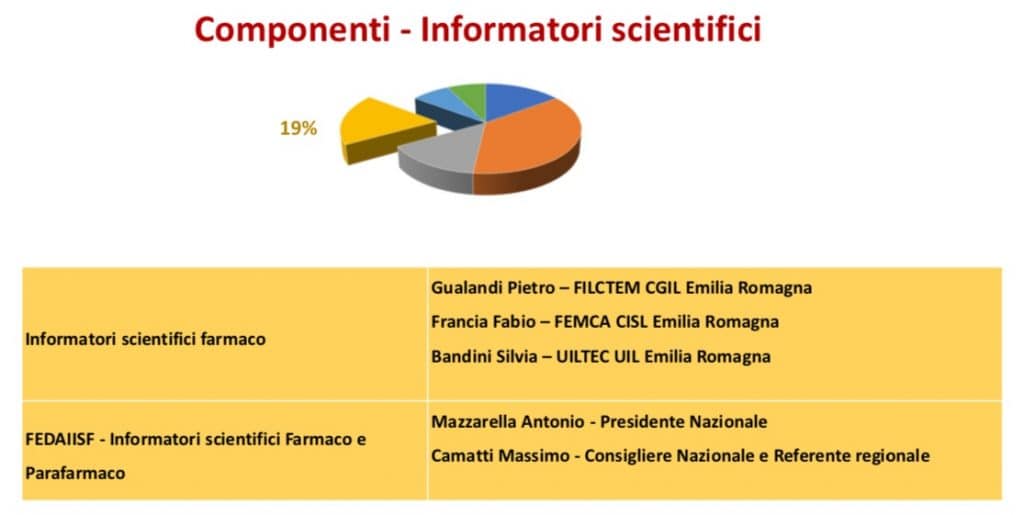
E’ importante sottolineare il fatto che questo portale varrà per tutta la regione ed è previsto che entri in funzione nei primi mesi del 2020.
We consider the outcome of the meeting to be positive and have parted ways with the commitment that the Working Group will meet again by mid-November to evaluate together and possibly approve a document that addresses the technical and practical provisions of the DGR 2309/2016 that better clarify the aspects of meeting management both at a territorial level (GPs, PLS, health houses) and at the hospital level.
Riportiamo il resoconto dell’incontro:
Brief report of the meeting Scientific Information Working Group –
October 21, 2019
The first meeting of the WG coordinated by Dr. Solfrini opens with the presentation of the members and their roles.
- identify homogeneous operating methods for the application of the DGR in the individual healthcare companies with the adoption of common tools, possibly electronic but simple to apply;
- evaluate opportunities and tools to track and enhance information activities on medical devices;
- share analyzes and proposals relating to the promotion of supplements in public health facilities.
with the intention of developing them in a limited number of meetings.
It is reiterated that the interest of the region is to guarantee transparency and traceability for the benefit and enhancement of the respective roles and the commitment dedicated to these activities by all the professionals involved, avoiding that the deliberated procedures determine an increase in administrative tasks or avoidable compilers.
The members of the regional part undertake to prepare an easy-to-fill electronic application which will be made available in all companies within the first months of 2020, maintaining the paper filling in the transition period.
It should be remembered that the only figure who has specific rules for defining the role and access is that of the drug scientific representative (Legislative Decree 219/2006), the other figures do not have supporting legislation and could be traced back to the bearers of interest or promoters.
On the subject of Medical devices, it is proposed to distinguish the profile of those who carry out training activities from that of information.
Related news: Emilia-Romagna.dgr-2309-2016
ANAC. Guidelines Code of Conduct SSN. for ISF p. 10 (bottom of page)
