Despite not having a Register or Order, the profession of the Scientific Drug Representative (ISF) is one of the most regulated professions that exists, in fact it must comply with European Directives, Italian State Laws, Guidelines of the Conference of Regions and Provinces Autonomous, Regional Regulations.
 Let's do some history on the reason for this excess of rules and regulations.
Let's do some history on the reason for this excess of rules and regulations.
With the pharmaceutical industry also came the need to make pharmaceutical products known to prescribers in general and doctors in particular. Thus was born the figure of the propagandist (as he was called then) who went to hospitals and surgeries to make medicines known on behalf of the industry he represented.
But as the industry grew with its growing portfolio of products, the potential ethical conflict of making money selling health-related products became increasingly clear. George Merck expressed himself on the subject in 1950: “We should never forget that medicine is for people. It's not for profits. Profits come as a result, and if we remember this lesson, they won't stop being there. The better we remember it, the greater the profits”. This industry involving the "public" required oversight, and drug regulations required by governments on both sides of the Atlantic increased.
The Cassation has also declared in a series of judgments that: “The consumption of medicines is not regulated by the criterion of pleasure, but by that of utility, mediated by the medical profession, so that doctors are the recipients of a specific form of advertising which aims not at abstractly advertising the product by praising its virtues or pleasantness view of the package, but to inform them of the nature and pharmaceutical uses of the product, in which cases it is indicated, in which it is not and in which it is even harmful (see Cass. n. 25053 of 2006; see also v. Cass. n. 8844 of 2014; Cass. n. 2349 of 2013; Cass. n. 5494 of 2013)"
 The need arose to set rules with the aim of avoiding conflicts of interest as much as possible, making scientific information dependent on a "scientific service" independent of marketing and making it qualified by a degree suitable for scientific representatives of the drug.
The need arose to set rules with the aim of avoiding conflicts of interest as much as possible, making scientific information dependent on a "scientific service" independent of marketing and making it qualified by a degree suitable for scientific representatives of the drug.
On August 5, 1978 the law was promulgated Law n.484 which lays the foundations for a regulation on scientific information and the advertising of medicines which was then regulated in the subsequent regulation which took place with the law n.833 of 1978, which establishes the SSN, which in art. 29 says that a State law must be implemented “la regulation of the scientific information service on medicines and of the activity of scientific representatives”, which was followed by various implementing ministerial decrees.
A few years later, the European Commission elaborated a series of Directives which concerned the homogenization of the legal provisions, concerning scientific information, to be applied in the individual countries making up the Community (Directive n. 92/28 EEC, transposed in Italy with the Legislative Decree 30 December 1992, no. 541, outlined the professional figure of the ISF). The Legislative Decree 541/92 established that the ISF had to be in possession of one of the degrees that the Legislative Decree itself listed. The Decree also provided for an amnesty for non-graduated ISF at the time of publication of the Decree in the Official Gazette.
 Coming to today, following the European Directives 2001/83/CE and 2003/94/CE, the Legislative Decree 24 April 2006, n. 219 that atarticle 122 ("Requirements and activities of scientific representatives"), reformulated the content of the activity of scientific representatives, in particular in paragraph 2 it says: "Without prejudice to the situations regularly in place on the date of entry into force of this decree, scientific representatives must be in possession of a university degree…” and lists the degrees suitable for the activity. Legislative Decree 219/06 in art. 158 repeals the previous Legislative Decree 541/92.
Coming to today, following the European Directives 2001/83/CE and 2003/94/CE, the Legislative Decree 24 April 2006, n. 219 that atarticle 122 ("Requirements and activities of scientific representatives"), reformulated the content of the activity of scientific representatives, in particular in paragraph 2 it says: "Without prejudice to the situations regularly in place on the date of entry into force of this decree, scientific representatives must be in possession of a university degree…” and lists the degrees suitable for the activity. Legislative Decree 219/06 in art. 158 repeals the previous Legislative Decree 541/92.
The question we are frequently asked is: which amnesty should be considered, that of 1992 or that of 2006?
Some maintain that in 2006 there should have been nothing to remedy since the anomalous situations had already been remedied by the 1992 Decree. Others maintain that the 1992 Decree was repealed, therefore the 2006 Decree, which starts with a new amnesty, applies.
The answer is given to us by AIFA, the drug regulatory body.
As it is known "within the month of January of each year, each pharmaceutical company must communicate to AIFA the list of scientific representatives employed during the previous year, with the indication of the qualification and the type of employment contract with the pharmaceutical company", etc..
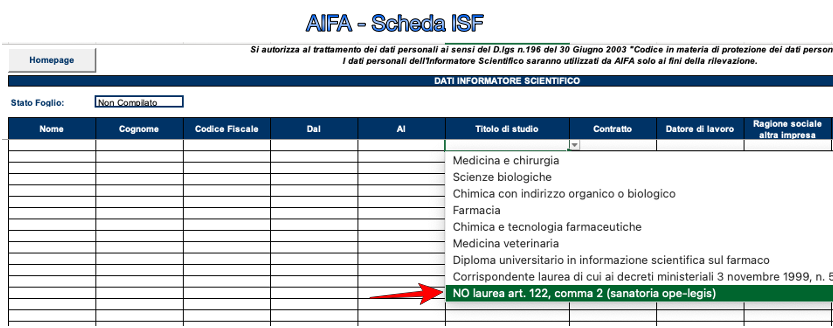 AIFA has prepared a pre-formatted form for the companies for the ISF to be filled in where under the heading "degree” a menu opens with answers already entered to be ticked by the same company: as regards the amnesty, the box to be ticked is “NO degree art. 122, paragraph 2 (amnesty ope legis)“. It is clear that AIFA itself refers to Legislative Decree 219/06 which is the text. Equally evident is that those who benefited from the amnesty of 1992 will continue to be healed.
AIFA has prepared a pre-formatted form for the companies for the ISF to be filled in where under the heading "degree” a menu opens with answers already entered to be ticked by the same company: as regards the amnesty, the box to be ticked is “NO degree art. 122, paragraph 2 (amnesty ope legis)“. It is clear that AIFA itself refers to Legislative Decree 219/06 which is the text. Equally evident is that those who benefited from the amnesty of 1992 will continue to be healed.
Of course then it's up to the ISF to prove that he was working with a pharmaceutical company on prescription drugs at a date before 2006.
Equally obviously the regulatory rules concern “medicines which can only be supplied upon presentation of a medical prescription or which contain psychotropic or narcotic substances" (art. 115, Legislative Decree 219/06)
Anyone who has parapharmaceuticals, supplements, nutraceuticals, dermocosmetics, devices, milk for babies, etc., has no legal constraints for which therefore no specific title is required.