The Italian Medicines Agency has published the first Pharmacovigilance Report on COVID-19 vaccines, which will be published on a monthly basis. The data collected and analyzed concern reports of suspected adverse reactions  registered in the National Pharmacovigilance Network between 27 December 2020 and 26 January 2021 for vaccines in use in the ongoing vaccination campaign: Comirnaty by Pfizer/BioNTech (authorized since 22/12/2020 and used since 27/12/2020) and COVID-19 Vaccine Moderna (authorized since 07/01/2021 and used since 14/01/2021).
registered in the National Pharmacovigilance Network between 27 December 2020 and 26 January 2021 for vaccines in use in the ongoing vaccination campaign: Comirnaty by Pfizer/BioNTech (authorized since 22/12/2020 and used since 27/12/2020) and COVID-19 Vaccine Moderna (authorized since 07/01/2021 and used since 14/01/2021).
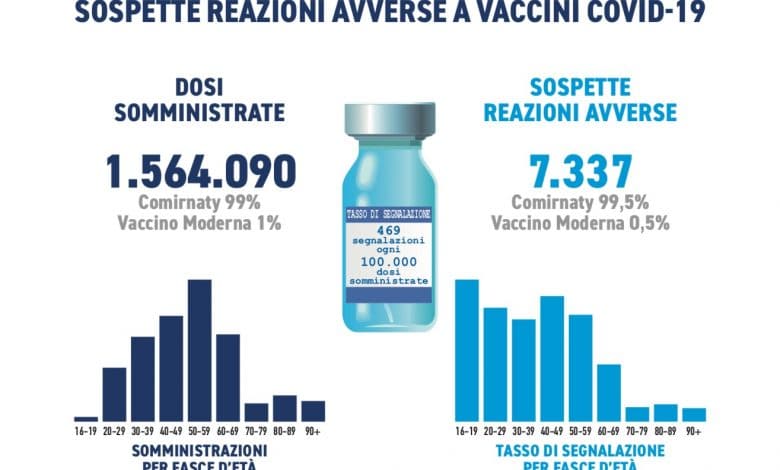
The reports mainly concern the first dose of the Comirnaty vaccine (99%), which was the most used and only to a lesser extent the Moderna vaccine (1%).
They were received during the period in question 7,337 reports out of a total of 1,564,090 doses administered (reporting rate of 469 per 100,000 doses), of which the 92.4% refer to non-serious eventssuch as injection site pain, fever, asthenia/fatigue, muscle aches. Headache, paraesthesia, dizziness, drowsiness and taste disturbances have also been observed with Comirnaty, while with the Moderna vaccine, nausea and abdominal pain.
Less frequent are other local reactions and widespread joint pain. As expected, fever was reported more frequently after the second dose than after the first.
The events reported mainly occur on the day of vaccination or the day after (85% of cases).
Of the 7.6% of reports classified as "serious”, for which the evaluation of the causal link with vaccines is underway, three out of four have not required specific intervention in the hospital setting.
During the period they were also reported 13 deaths occurred in the hours following vaccination that, in the most detailed and complete reports of data, were not related to vaccination and are largely attributable to the basic conditions of the vaccinated person.
The analyzes conducted on the data acquired so far therefore confirm a good safety profile of these two mRNA vaccines. The large number of reports does not imply that unexpected critical issues have emerged, but it is an indication of the high capacity of the pharmacovigilance system in monitoring safety.
Related news: European Parliament: “Vaccines against covid-19: ethical, legal and practical considerations





