
Sandwiched between doctors, pharmacists, sales reps (those who skip the line at the clinic...) and pharmaceutical companies, what do we need to know in order to consciously choose how to treat ourselves? They are convenient and Almost always the same as those with the patent, yet we consume less than in other countries
by Cinzia Lucchelli – 06 February 2015 – MANTOVA GAZZETTA
"Non-replaceable drug, doctor incredulous about the virtues of generics". You also read this on the stamps of doctors defending brand name medicines against their equivalents. In addition to doubts about the orthodoxy of the form, the photo of a recipe with this inscription, posted on Facebook, has caused a hornet's nest of reactions. Are generic painkillers, antibiotics, cholesterol lowering drugs as effective as the originals? The debate is open. In Italy, consumption is constantly increasing but is struggling to establish itself. «Today out of 100 packages sold, 20 are generics – he says Enrique Häusermann, president of AssoGenerici – as far as expenditure is concerned, out of 100 euros the 10% is due to generics». But we are behind Europe.
This despite the fact that the equivalents cost at least 20% less and despite the fact that the medicines available in Italy are the same as prescribed in Europe (they are produced by multinationals). Where, then, do delay and distrust come from? And above all, caught between doctors, pharmacists, scientific representatives (those who skip the line at the clinic...) and pharmaceutical companies, what do we need to know in order to choose with awareness how to treat ourselves?
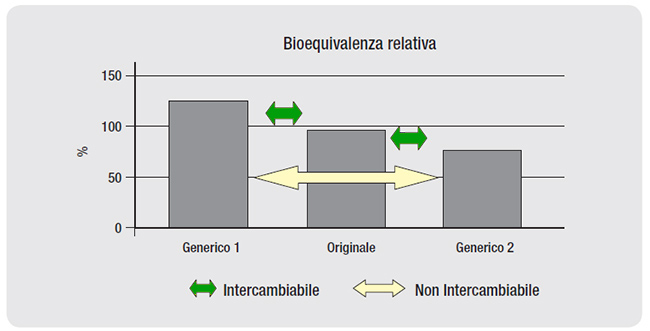 Same formula. A generic drug is a copy of a branded drug that has been on the market for years and is no longer covered by a patent. Contains the same active ingredient, in the same amount. It shares its pharmaceutical form, route of administration (for example tablets), dosage. Furthermore, it is a bioequivalent of the reference medicinal product, i.e., as the Italian Medicines Agency writes, with the same dose the blood concentration profiles of the two medicinal products with respect to time «are so similar that it is unlikely that they will produce significant differences in the effects of efficacy and safety".
Same formula. A generic drug is a copy of a branded drug that has been on the market for years and is no longer covered by a patent. Contains the same active ingredient, in the same amount. It shares its pharmaceutical form, route of administration (for example tablets), dosage. Furthermore, it is a bioequivalent of the reference medicinal product, i.e., as the Italian Medicines Agency writes, with the same dose the blood concentration profiles of the two medicinal products with respect to time «are so similar that it is unlikely that they will produce significant differences in the effects of efficacy and safety".
Different prices. The same formulas, therefore, but different prices. This is because when a company develops a drug, it protects it, in the European Union, with a 20-year patent. Period during which it can sell the drug at a price that allows it to recover the investment for research and studies necessary to demonstrate efficacy and safety. Companies that copy and produce the drug as a generic when coverage has expired can lower the price because they no longer have to invest in research and studies.
The market in Italy. In 2012, according to the Osmed National Report, equivalents represented 13.4% of total expenditure (they were 9.5% in 2011). Broadly speaking, the consumption of expired patent medicines represents 62% of the consumption in the agreed assistance regime; 37.7% of the agreed net expense. Consumption increases, yet, it doesn't take off. Especially in comparison with England, France and Germany.
Generic in name only, The distrust perhaps already arises from the name. “People know them as 'generics', a term that sounds bad,” he says Silvio Garattini, director of the Mario Negri Institute in Milan – but what is generic is not the drug, but its name». Name, in fact, which is neither chemical nor fantasy. Since 2005 generics in Italy are called equivalents.
 The doubt of the excipients. Another reason for caution concerns excipients, the only substances that change between branded and generic drugs. They have no pharmacological activity, they serve to make the active ingredient administrable. But they may have relevance for the safety of the medicine: medicines with sucrose must be carefully administered to diabetics, those with lactose are not suitable for those who are intolerant. A doctor who knows the patient knows how to behave.
The doubt of the excipients. Another reason for caution concerns excipients, the only substances that change between branded and generic drugs. They have no pharmacological activity, they serve to make the active ingredient administrable. But they may have relevance for the safety of the medicine: medicines with sucrose must be carefully administered to diabetics, those with lactose are not suitable for those who are intolerant. A doctor who knows the patient knows how to behave.
Delay due to patent. The long patent times, then, are among the causes of the delay of generics in Italy. In 1991, a Complementary Protection Certificate was introduced which extended the exclusivity of the exploitation of a molecule up to 38 years. Measure exceeded the following year by an EEC regulation which granted the extension of the patent for a maximum of five years. But meanwhile the 80% of the active ingredients on the market had obtained a longer coverage. By postponing the entry of generics and the consequent savings for the national health system.
When the doctor decides. Branded or generic? In some cases it chooses the doctor. When treating a patient for the first time, the prescription can only indicate the active ingredient and the pharmacist will have to provide the generic with the lowest cost (the State, among equivalent packs, reimburses the cheapest one and if the patient asks for a branded product pay the difference). But the doctor can also indicate the brand name of the drug. If he then adds "non-replaceable", the pharmacist can only supply the "branded" drug.
_______________________________________________________________
Ed.: define the Scientific Representatives of the drug: "those who skip the line at the clinic…” it implies an implicit judgement, indeed a prejudice. But we don't want to enter into a controversy with those who have pre-judgments. Nor do we want to go into the merits of the various "pro generic" chapters set out because it would require too much space.
However, since the Osmed 2012 data are mentioned (evidently they are convenient for Dr. Häusermann), we inform you that the OsMed report 2014 and literally says: "Expired patent medicines now represent more than half (51.1%) of approved pharmaceutical expenditure, up on 2013 by +6.6%, and 70.4% of total DDDs, up on 2013 by +11.9% . The percentage spent on generic medicines (drugs based on active ingredients with expired patents, excluding those that have enjoyed patent protection) was equal to 28.8% of the total of expired patent medicines (Table 12). Overall, the top twenty patent-expired active ingredients represent approximately 50% of the consumption, in terms of DDD, of all patent-expired drugs; pantoprazole and lansoprazole, which dropped to second position in 2014, were the active ingredients with expired patents at the highest expense, with respectively 217.5 and 189.2 million euros”.
AIFA also states: For land active substances imported from non-EU countries That "they will be able to enter Italy to be used in the production of medicines for human use” underlines Isabella Marta, AIFA Raw Materials Inspection Management Unit, “only if accompanied by a certification from the competent authority of the country of production, which certifies its equivalence to the quality standard valid in Europe, or if accompanied by a GMP certificate from a European country. This measure is to protect the quality of medicines produced and marketed in Italy. Furthermore, AIFA will be able to inspect producers or importers of excipients, in case of suspected non-compliance with the expected quality requirements and, possibly, if deemed appropriate in the context of collaboration with the Ministry of Health, distributors of active substances located in the territory national or established in third countries” ( Guide to the implementation of Legislative Decree n. 17 of 19 February 2014, as regards active substances and excipients )
It should be noted that the FDA has set up a task force specifically responsible for verifying the quality of the active ingredient and the production method on site since it does not absolutely trust the certification from the competent authority of the country of production e publish “Orange Book” in which it certifies the real equivalence between generic and branded drugs.
Everyone draws the conclusions they want.
For the sake of truth.
Related news: Generic drugs, Osmed: the Italians are starting to convince






