
Variation of generic drug and adherence to therapy
Medication adherence decreases by 28% if half of generic drug prescriptions are replaced with another equivalent
 During the conference organized by the National Women's Health Observatory (Onda), in collaboration with DOC Generici, and held in Milan at the NH Hotel Touring in via Targhetti 7, the results of a study conducted on a sample of over 14,500 patients of the ASL of Bergamo and Pavia, which analyzed 6 pathologies/therapeutic areas - diabetology, cardiology, dyslipidemia, rheumatology, psychiatry and hypertension - to investigate the relationship between the switch between equivalent drugs and adherence to the doctor's prescription , thus studying the effects of replacing a generic drug with another equivalent in terms of adherence and persistence to the current therapeutic treatment.
During the conference organized by the National Women's Health Observatory (Onda), in collaboration with DOC Generici, and held in Milan at the NH Hotel Touring in via Targhetti 7, the results of a study conducted on a sample of over 14,500 patients of the ASL of Bergamo and Pavia, which analyzed 6 pathologies/therapeutic areas - diabetology, cardiology, dyslipidemia, rheumatology, psychiatry and hypertension - to investigate the relationship between the switch between equivalent drugs and adherence to the doctor's prescription , thus studying the effects of replacing a generic drug with another equivalent in terms of adherence and persistence to the current therapeutic treatment.
The data were discussed by Prof. Alberico Catapano, President of the European Society of Atherosclerosis, Prof. Claudio Mencacci, President of the Italian Society of Psychiatry, Prof. Enrico Agabiti Rosei, President of the European Society of Hypertension, Prof. Carlomaurizio Montecucco, SC Director of Rheumatology, San Matteo Polyclinic of Pavia, Prof. Alberto Margonato, Director of the Clinical Cardiology Division, IRCCS San Raffaele Hospital in Milan, Prof. Roberto Trevisan, Director of the Endocrine Diseases and Diabetology Unit of the 'AO Pope John XXIII of Bergamo and Prof. Giorgio L. Colombo of the Department of Pharmaceutical Sciences of the University of Pavia. The work of the conference was introduced and conducted by Dr. Nicoletta Orthmann, medical-scientific coordinator of the Onda.
Last February we presented the data of one of our surveys, conducted on a sample of 445 women, with the aim of exploring the methods of approaching generic drugs and the degree of satisfaction, with particular reference to the consequences of a possible switch from a drug to another on the continuation of therapy. – stated Nicoletta Orthmann, Onda's medical-scientific coordinator - Three women
out of four reported that substituting one generic for another was a problem, which in 19% of cases resulted in lower adherence to therapy (errors of hiring, suspension or termination). The preliminary results of the study that we are presenting today constitute the 'evidence based' confirmation of what emerged in our investigation: the more the number of substitutions between generics increases, the lower the adherence to the therapeutic program in progress. For chronic pathologies, such as those taken into consideration in the research, it is, however, crucial to maintain therapeutic continuity, even after the achievement of positive results, to ensure greater efficacy and safety of the treatment and reduce the risk of complications and hospitalization
The data presented at the Milanese conference follow a previous study published in PlosOne in 2013, which compared generic and branded medicines with expired patents, from which the clinical equivalence between brand and generic had emerged and in which persistence and compliance were higher for the equivalent drug.
The study presents several aspects worthy of attention. – declared Professor Enrico Agabiti Rosei, President of the European Society of Hypertension – First of all data confirm that the practice of substituting a generic with another equivalent drug is very commonaffecting more than half of the patients. Furthermore, the 'switch' often occurs repeatedly, with a frequency between one change every 3 and one every 5 prescriptions, in relation to the different therapeutic areas. The results also show that in patients with repeated 'switches' there is a clear fall in treatment adherence and persistence, and this draws attention to the possible risks associated with frequent changes in the drug dispensed.
In all 6 areas examined by the study, a progressive trend of reduction in compliance was found following a drug change: on average, if one generic prescription out of two is replaced with another equivalent, for dyslipidemia and diabetes shows the highest percentage of decrease in adherence (48% and 36% respectively), followed by rheumatology (21%) and psychiatry (19%) and hypertension (10%).
The greater the number of generic drug substitutions, the lower the compliance.
 Adherence to therapy and the horizontal replacement of equivalent medicines are inversely proportional: as the switch from one generic to another of the same molecule increases, in fact, the patient's adherence to the therapeutic prescription decreases, with a value equal to 28% if the substitution affects half of the prescriptions. The decline in adherence to drug prescription therefore implies a lower efficacy and safety of therapeutic treatments, with a consequent increase in the risk of complications and a worsening of the patients' health conditions.
Adherence to therapy and the horizontal replacement of equivalent medicines are inversely proportional: as the switch from one generic to another of the same molecule increases, in fact, the patient's adherence to the therapeutic prescription decreases, with a value equal to 28% if the substitution affects half of the prescriptions. The decline in adherence to drug prescription therefore implies a lower efficacy and safety of therapeutic treatments, with a consequent increase in the risk of complications and a worsening of the patients' health conditions.
Even in the area of psychiatry – claimed Professor Claudio Mencacci, President of the Italian Society of Psychiatry and Director of the Department of Neurosciences of the Fatebenefratelli Hospital in Milan – the study demonstrated that as horizontal substitution between generic drugs increases, adherence and persistence to therapy decrease. It is essential, as demonstrated by the AIFA Guidelines of 2014, that, regardless of the antidepressant used, the treatment lasts at least 6 months in patients suffering from depression, by virtue of the high risk of recurrence, to which a large part of the economic costs are attributed and social aspects of the pathology. Previous observational studies have shown that almost 50% of patients on therapy discontinue treatment in the first 3 months and 70% in the first 6 months. It is therefore important to reduce the factors that can affect adherence to treatment and also those biological factors that can interfere with the good clinical outcome achieved.
Therefore, the advice is to always keep the same 'generic brand' with which the treatment was started and the positive results were achieved.
Bioequivalent drugs do not present criticalities in clinical use both by specialists and by general practitioners – said Professor Alberico Catapano, President of the European Atherosclerosis Society – demonstrating, in a large series of studies, fully equivalent to the so-called 'branded' in clinical efficacy, measurable in the population treated also through studies of 'real life' use. They also represent an opportunity to contain pharmaceutical expenditure, without prejudice to the concept of citizens' freedom of choice. There remain some 'psychological' barriers that originate from the incomplete understanding by healthcare professionals (doctors, pharmacists, etc.) of the concept of bioequivalence, in addition to the non-uniformity of the packaging which, in the case of elderly people, can 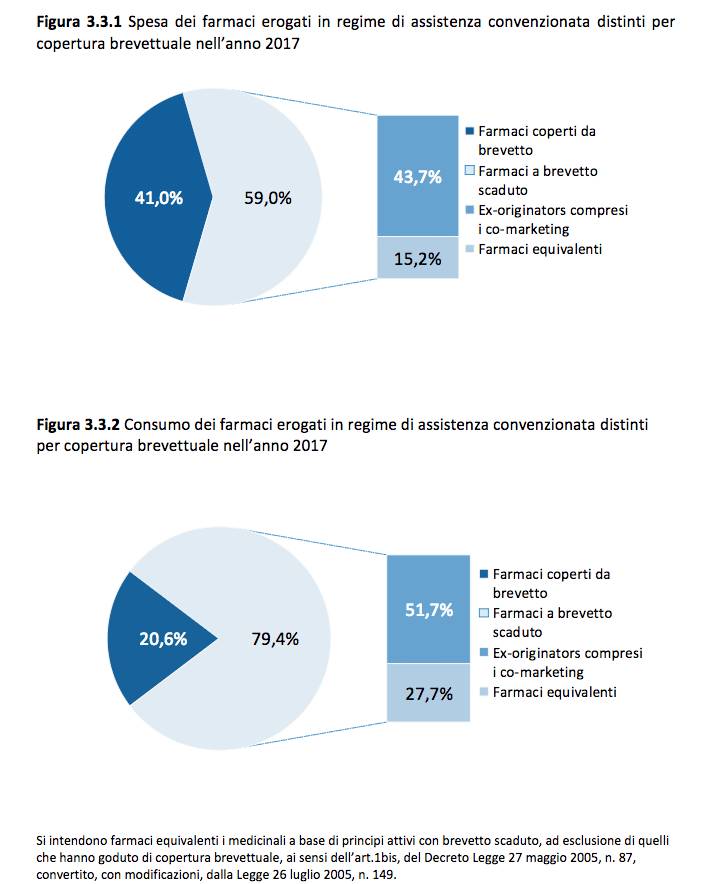 be a problem and lead to relevant therapeutic discontinuities.
be a problem and lead to relevant therapeutic discontinuities.
The entry of equivalent (or generic) medicines into the world pharmaceutical market is a phenomenon of considerable interest in economic and social terms, which has significantly changed both company strategies and the behavior of all the players involved in healthcare spending and in the prescription of drugs. – underlined Professor Giorgio L. Colombo of the Department of Pharmaceutical Sciences, University of Pavia – Among the numerous "cost-containment" tools adopted by all modern healthcare systems, those that aim not so much to block consumption, impose discounts or cut prices, but to increase the allocative efficiency of the system, deserve particular interest. restoration of market competitiveness obtainedto stimulating the "price competition" of the producers, as in the case of equivalent drugs.
OFF patent medicines (patent expired) represent, to date, almost half of territorial consumption in Italy, even if the greatest prescription is still concentrated on branded products, unlike what happens in other European countries where favors the pure equivalent drug (unbranded). However, the presence of a significant share of the market for pure equivalent medicines (unbranded) is a necessary condition for price competition in the sector to unfold and companies, upon expiry of the patent, begin to compete on price with strong reductions and with simultaneous benefits for the public health system.
Furthermore, when the patient buys the generic branded product, he must pay a co-payment fee at his expense, while this does not happen for the pure generic product, which is completely paid for by our public health service.
In conclusion – reiterated Professor Alberto Margonato, Director of the Clinical Cardiology Division, IRCCS San Raffaele Hospital, Milan –
once a generic drug has been prescribed, the doctor must ensure that it is not replaced with an equivalent drug from a different manufacturer.
Variation in the characteristics of the packaging, shape and color of the tablets can invalidate these results.
Related news: Replacement in the pharmacy, by pharmacists commitment to generic continuity to promote adherence
Generics and chronicity, alert from UniPv survey: adherence drops if replacement increases





