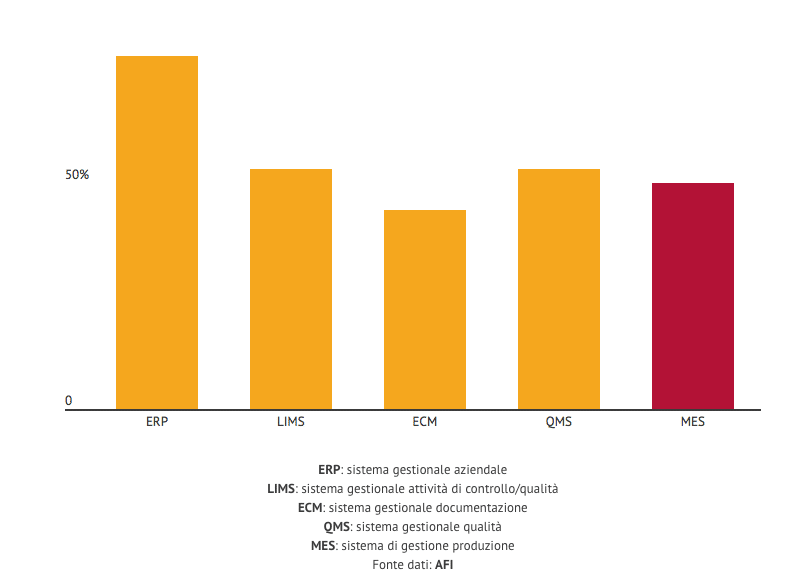Is the Italian pharmaceutical industry ready for 4.0? The answer is almost
The results of a survey involving around fifty pharmaceutical companies have been presented: only half are ready for 4.0
Wired – 9 May 2018 – by Riccardo Saporiti
It is the most important pharmaceutical sector in Europe, second only to the Germany, with an export that exceeds 70% of the total production. But the Italian pharmaceutical sector is ready to move towards industry 4.0? The answer is that there is still much work to be done. At least this is what emerged from a survey conducted in 2017 from Pharmaceutical industry association (Afi).
The results of which were disclosed this morning at Milan as part of the presentation of the sixth edition of Pharmintech, life science fair scheduled at Bologna from the 10 to the 12 April 2019. The result is that only a percentage of industries between the 50 and the 60% is ready for the leap to digitization. This can be understood by looking, for example, at these numbers:
The most interesting element is related to monthi.e. the production management system. “It is about that infrastructure that will allow integration between all the machines.
And we are stuck at 50%”, he stressed Alberto Bartolini, coordinator of the Afi supply chain area.
Now, it is necessary to specify that of the 200 companies to which the survey was submitted, only about fifty responded. Therefore, without having a production management information system, I am, with certainty, only the 12,5% of Italian pharmaceutical companies. Unless the proportion is respected even among those who have not answered Afi's questions.
 We must also take into account the fact that the request was for IT managers to respond. But only in the 5% of cases this has occurred. The fact remains that, explained Bartolini, “from a 4.0 point of view, all these indicators must reach the 100%”. Both among those who responded and among those companies that didn't decide to do so. But that is not all.
We must also take into account the fact that the request was for IT managers to respond. But only in the 5% of cases this has occurred. The fact remains that, explained Bartolini, “from a 4.0 point of view, all these indicators must reach the 100%”. Both among those who responded and among those companies that didn't decide to do so. But that is not all.
Only the 56% of the companies that provided an answer stated that their systems are validatable. That is, they have the characteristics necessary to be usable in accordance with the law. “This means that validation today comes with paper supports and procedures. And that in the future it will be necessary to invest to replace these systems". Again, only the 58% performs a data integrity verification process. So is there a safety problem in pharmaceutical companies? “No, because those who do not carry out these processes digitally do so with paper procedures”, he stressed George Bruno, ad of recipharm and Vice President AFI.
The problem, in other words, is digitization. And it is all the more serious the more one considers that "in the pharmaceutical sector the choice of 4.0 does not exist: it is an obligation", emphasized the president of Pharmintech Sergio Dompé. Ultimately, concluded Bartolini, “There is a lot of room for improvement. Which, however, must be launched in a very short time, because the new processes are arriving".
Related news: Scaccabarozzi: we are already industry 4.0. “We have 15,000 new drugs under development and 7,000 in clinical trials.”
Dompé, investing in 4.0 is a must for the pharmaceutical sector
Ed: Dr. Scaccabarozzi, President of Farmindustria, speaking at the Medical Science Festival in Bologna, said "We are already industry 4.0" and added: "We have 15,000 new drugs under development and 7,000 in the clinical trial phase".
Now, what emerged from a survey conducted in 2017 by the Pharmaceutical Industry Association (AFI) shows that only a percentage of industries between 50 and 60% is ready for the leap towards digitalisation.
At the same time, EMA, the European Medicines Agency, in its report, certifies that in 2017 it approved 92 drugs for the European Union (28 countries), of which only seven are new active substances.
We seem to catch some inconsistencies, the AFI data denies the statements of Dr. Scaccabarozzi as well as the EMA data which speaks of seven new active substances throughout Europe. Is it possible that none of the seven thousand drugs undergoing clinical trials has already reached the EMA?