After 8 years of absence, the Ministry of Health, General Directorate of digitization, health information system and statistics, published the "Report on the country's health status 2017-2021”. The Report addresses above all the questions that have arisen in recent years. Such as, for example, the waiver of care and the new guarantee system of essential levels of assistance (Lea) and it serves as an organic tool for assessing the health objectives achieved and the strategies put in place to achieve them. It is an undoubtedly positive result that allows us to have an indispensable overview of the NHS to address critical issues such as inequalities in assistance” comments Tonino Aceti, president of healthequity.
We reproduce an excerpt from the Report in its entirety.
The World Health Organization (WHO) defines medicines that meet health needs as “essential”. 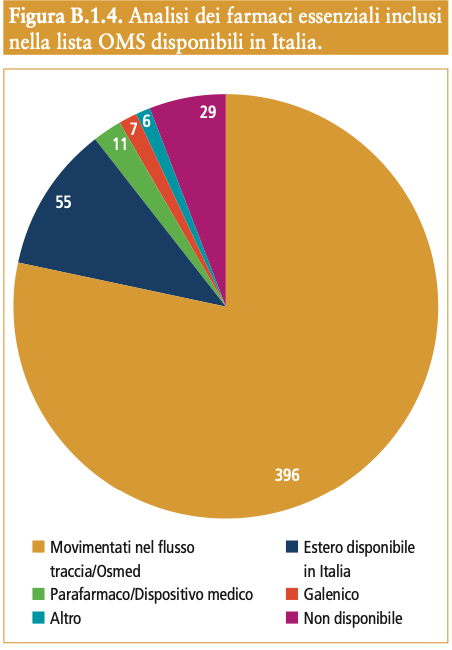 priorities of a population, selected with due regard to disease prevalence and relevance to public health, evidence of efficacy and safety, and comparative cost-effectiveness, which should be available in appropriate, quality-assured dosage forms and at affordable prices. From the analysis carried out2, more than 90% (n. 475) of the drugs included in the 22nd edition of the list drawn up by the WHO is available in Italy of which the 80% is intercepted by the Pharmaceutical Data Flows (Traceability Flow and Osmed Flow) , 12% is represented by imported drugs used in hospital facilities4, 3% by Parapharmaceuticals and/or Medical Devices and 1% is represented, respectively, by galenic drugs and others5.
priorities of a population, selected with due regard to disease prevalence and relevance to public health, evidence of efficacy and safety, and comparative cost-effectiveness, which should be available in appropriate, quality-assured dosage forms and at affordable prices. From the analysis carried out2, more than 90% (n. 475) of the drugs included in the 22nd edition of the list drawn up by the WHO is available in Italy of which the 80% is intercepted by the Pharmaceutical Data Flows (Traceability Flow and Osmed Flow) , 12% is represented by imported drugs used in hospital facilities4, 3% by Parapharmaceuticals and/or Medical Devices and 1% is represented, respectively, by galenic drugs and others5.
Although the drugs included in the WHO list, including therapeutic alternatives, represent only the 30% of the drugs present in the database of marketed drugs (total count n. 714 out of 2396 level V ATCs), they constitute the 74% of total consumption [in terms of defined daily doses (defined daily dose, DDD)], testifying to the fact that in Italy they are widely used, despite the wide availability of multiple active ingredients.
The 97% of drugs included in the EML list (Essential Medicines List) available in Italy is provided at the expense of the National Health Service (SSN), in line with the WHO definition of an essential drug; the remaining 3%6 is represented by devices and/or parapharmaceuticals paid for by the citizen. In the last 50 years in Italy mortality has clearly decreased, life expectancy has grown by 1 month every 4 and today Italy ranks among the first in the world for the average value of life expectancy, also thanks to the large availability and accessibility of medicines and vaccines, which contribute daily to the improvement of citizens' health.
It was observed that the time between the date of the European Commission's decision and the date of submission of the P&R request by the pharmaceutical company is, from 2018 to 2020, an average of 10 to 12 months for non-generic drugs and from 7 to 12 months for generic drugs, presenting a strong variability over time for both pharmaceutical categories.
The overall duration of the procedure - from the start date to the conclusion date, including the evaluation times by the Agency's advisory commissions (HTA Secretariat, Technical-Scientific Commission and Pricing and Reimbursement Committee) - decreased from 2018 to 2020, rising from 9 to 8 months on average for non-generic drugs. As regards the transmission time in the Official Gazette, from the date of conclusion of the proceeding up to the transmission in the Official Gazette of the related provision, a decrease was observed over time, going from just over 2 months in 2018 to 2 months in 2020, for both generic and non-generic drugs.
 The purpose of recognizing the requirement for therapeutic innovation is to guarantee homogeneous access at a national level, on the basis of the State-Regions agreement of 18 November 2010, for all those drugs indicated for the therapeutic areas in which there is actually a need important and/or dissatisfied clinician. This access is guaranteed through the inclusion of these drugs considered innovative in the Regional Hospital Therapeutic Handbooks. From an economic point of view, innovative drugs benefit from the suspension of the legal reductions of 5%+5%. The 2017 Budget Law (art. 1, paragraphs 397-408, of Law 232/2016) also provided for a specific loan for the purchase of innovative drugs, which resulted in the establishment of two funds ad hoc: a fund dedicated exclusively to the purchase of innovative oncological medicines, with an endowment of 500 million euros per year, and a fund for the reimbursement competition for the Regions for the purchase of the remaining classes of innovative medicines.
The purpose of recognizing the requirement for therapeutic innovation is to guarantee homogeneous access at a national level, on the basis of the State-Regions agreement of 18 November 2010, for all those drugs indicated for the therapeutic areas in which there is actually a need important and/or dissatisfied clinician. This access is guaranteed through the inclusion of these drugs considered innovative in the Regional Hospital Therapeutic Handbooks. From an economic point of view, innovative drugs benefit from the suspension of the legal reductions of 5%+5%. The 2017 Budget Law (art. 1, paragraphs 397-408, of Law 232/2016) also provided for a specific loan for the purchase of innovative drugs, which resulted in the establishment of two funds ad hoc: a fund dedicated exclusively to the purchase of innovative oncological medicines, with an endowment of 500 million euros per year, and a fund for the reimbursement competition for the Regions for the purchase of the remaining classes of innovative medicines.
The increase in the share of the 2021 indistinct fund compared to 2019, including the bonus shares, added to the additional resources made available by national regulations (2021 Covid-19 resources, 2020 Covid-19 provisions to be used in 2021, further state contribution for the coverage of the Covid-19 costs deriving from the payback), was substantially sufficient to cover the increases in costs recorded in the same period 2019-2021.
Finally, with reference to the year 2021, the results of the data for the fourth quarter of 2021 show a general worsening of almost all the Regions, in particular of the Puglia Region, which records a deficit before coverage equal to 239 million euros. Lazio: –83.5 million euros; Abruzzo: 0.7 million euro; Molise: -60 million euro; Campania: -69.5 million euro; Puglia: –239 million euro; Calabria: +27 million euro; Sicily: 0.5 million euro.
Related news. Ministry of Health. Report on the health status of the country