Poor oncology drugs, both in terms of efficacy and safety, would have been used in at least 16 Italian hospitals. Numerous patients (including children) would thus have taken medicines imported from theIndia, low cost and not approved in Europe. A procedure put into practice by seven territorial health authorities, while the number of hospitals could be higher. An investigation by Bureau of Investigative Journalism, "Politic" of which we report below the text
 At least 16 hospitals in Italy have treated cancer patients with a poor-quality imported drug that isn't approved for use in the EU. reveals the Bureau of Investigative Journalism (TBIJ) and POLITICO.
At least 16 hospitals in Italy have treated cancer patients with a poor-quality imported drug that isn't approved for use in the EU. reveals the Bureau of Investigative Journalism (TBIJ) and POLITICO.
Gaps in national and European regulation have allowed Italian hospitals to legally request shipments of Celginase, a low-cost cancer drug that has proven to be substandard, even when better alternatives were available.
Neither the Italian drug regulatory authority nor the country's health ministry are responsible for checking the quality, efficacy or safety of this drug before allowing it in Italian hospitals. Nor is this within the mandate of the European Medicines Agency (EMA).
Hundreds of vials of the drug have arrived from India over the past seven years. It is not known how many cancer patients may have experienced negative side effects or decreased chances of remission. Many vials can still be found on hospital shelves today.
Celginase is a brand name for asparaginase, a drug used to treat acute lymphoblastic leukemia, the most common form of childhood cancer. Celginase is manufactured and approved in India and costs a small fraction of the price of the "gold standard" brand: just €13 per vial compared to around €2,500. Published academic studies have found that it does not meet minimum manufacturing standards or consistently reach the clinical activity threshold for cancer treatment.
In January, TBIJ and STAT disclosed that brands of Poor quality asparaginase, including Celginase, has been shipped to more than 90 countries since 2016, putting an estimated 70,000 children worldwide at risk.
In 2018, an apparent nationwide shortage of Oncaspar, the "gold standard" asparaginase, led to children in Italy being given another brand, Aspatero, which was later deemed substandard. (The affected hospital said its application to import Aspatero was cleared by Italy's drug regulator and that not doing so would "reduce the children's chances of recovery." The drug regulator confirmed it had cleared the import.)
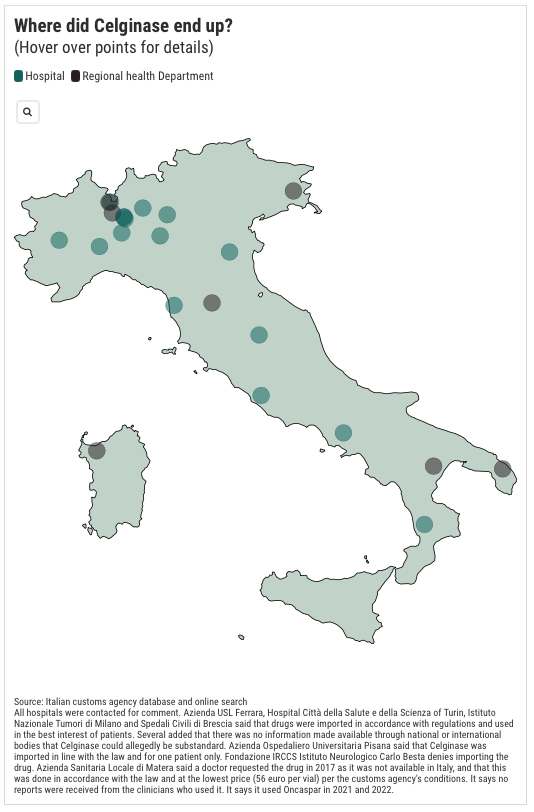
But TBIJ and POLITICO can now reveal that Celginase was bought by Italian hospitals even when the gold standard product was available, in some cases only this year. Additional records show that Celginase was also imported by seven Italian regional health departments, suggesting the actual number of hospitals using the drug could be much higher.
Informed consent documentation does not specify the brand of drug that will be given to patients or the countries where it is approved. Patients are left in the dark.
Lack of accountability
There are several types of asparaginase. "Native" asparaginase is produced by Escherichia coli (E. coli). Turning this bacterium into medicine is tricky, and even good quality native asparaginase can cause side effects, including severe allergic reactions.
For this reason, doctors prefer to use modified versions of asparaginase. These are less likely to cause allergic reactions but are much more expensive.
 Although Celginase and Oncaspar are both asparaginases, they are different: Celginase is native, Oncaspar is modified. Oncaspar has been approved for use in Europe since 2016 and is the brand name of asparaginase recommended as the first-choice treatment for acute lymphoblastic leukaemia.
Although Celginase and Oncaspar are both asparaginases, they are different: Celginase is native, Oncaspar is modified. Oncaspar has been approved for use in Europe since 2016 and is the brand name of asparaginase recommended as the first-choice treatment for acute lymphoblastic leukaemia.
In Italy, if a drug is in shortage, doctors and pharmacists can ask the Italian drug regulatory authority to authorize the import of the same drug from another country.
A different law allows for the importation of a medicine that is not approved in the EU but is found in other parts of the world, such as Celginase, for a single named patient if a doctor believes there is "no viable alternative therapy available". Because Celginase and Oncaspar are not exactly equivalent, doctors have been able to apply for Celginase to be imported even though Oncaspar is available.
Basically, this process bypasses the country's drug regulatory authority. In Italy, however, the authorization is granted by the health ministry's customs agency, which is only required to verify that the practices have been correctly completed by the hospital and that the drug arriving is the one requested.
It is not required by law to check the quality of imported medicines or to ask for data from the overseas regulatory authorities that approved them.
The drug was intended to save the lives of the children. Instead they are dying
Instead, the responsibility for these drugs lies with the individual doctor who requests them. The Italian Medicines Regulatory Agency said that doctors should use qualified and reliable import intermediaries who can guarantee the quality and safety of the drug, especially with suppliers in non-EU countries, and that doctors should obtain informed consent by providing the patient with complete, transparent and comprehensive information about the drug.
Through freedom of information requests to the health ministry's Italian customs agency, TBIJ and POLITICO found that at least 16 hospitals, including the Istituto Nazionale Tumori in Milan and San Camillo Forlanini in Rome, imported hundreds of vials of Celginase into the country over a seven-year period. (The Istituto Nazionale Tumori said the shortage required them to procure Celginase. It added that patients who received the drug are either responding to it or are in remission and that the Italian drug regulator has never provided information showing Celginase could be harmful. San Camillo Forlanini did not respond to a request for comment.)
Attilio Guarini, a hematologist and director of the Area Medica department, at the Iress Istituto Tumori Giovanni Paolo Il in Bari, which treats patients with acute lymphoblastic leukemia, says there is no clinical guide recommending doctors turn to a native asparaginase – such as Celginase – if Oncaspar is available.
Italy's medicines regulator told TBIJ it denies any responsibility for the "efficacy and safety" of medicines imported from non-EU countries that are not authorized in accordance with EU regulations.
The European Medicines Agency (EMA) also has no power over imports of unapproved medicines from third countries, according to its former boss Guido Rasi.
Most of the Celginase imported into Italy from India came from Switzerland. The Swiss customs agency said they did not have specific data on the products we asked for. He did not know whether Indian asparaginase imported through Switzerland might have gone to other EU countries besides Italy, but it is understood that celginase has not been used in Swiss hospitals.
Automatic translation from Bureau of Investigative Journalism (TBIJ) e Politic.
Note:
For Aifa "there is no feedback from the WHO or from national regulatory authorities of a low quality, dangerousness or ineffectiveness of pegaspargase or asparaginase-based medicines imported from India. The same Monza and Brianza Foundation for the Child and his Mother (MBBM), states that 'all patients treated with Aspatero and Oncaspar concluded the treatment phase in question without further significant adverse effects and continued regularly with the treatment envisaged in the subsequent phases'".
Also the Directorate-General for Welfare of the Lombardy Region in a note signed by ASST Cremona, ASST Spedali Civili, ASST Ovest Milanese, IRCCS Istituto Nazionale dei Tumori, ASST Papa Giovanni XXIII, IRCCS Istituto Neurologico Carlo Besta, ASST Sette Laghi and IRCCS Policlinico San Matteo, he reiterated that Asparaginase, of Indian production, was imported "only in the period in which there was a shortage of that of German origin (Medac), until then the reference drug. All this to guarantee an indispensable and irreplaceable drug in many oncohaematological chemotherapy protocols, in particular for Acute Lymphatic Leukemia. It has never been preferred for economic reasons. Furthermore, we would like to point out again that there is no feedback from the WHO, nor from national regulatory authorities of a low quality, dangerousness or ineffectiveness of pegaspargase or asparaginase-based medicines imported from India. The study that demonstrated a lower efficacy of Celginase compared to Oncaspar comes from Lombardy and dates back only to May 2022 ".
“All import procedures – concludes the note – strictly followed the provisions of the Ministry of Health legislation. As with all haematological cancer patients, rigorous follow-up procedures were followed to verify the efficacy of the therapies and any adverse effects. Most of the patients treated are cured or in remission of the disease”. (Source: Everyday healthcare)
Original article
Italian hospitals imported unapproved, substandard cancer drug
At least 16 hospitals in Italy have been treating cancer patients with a poor-quality imported drug that is not approved for use in the EU, the Bureau of Investigative Journalism (TBIJ) and POLITICO can reveal.
Gaps in national and EU regulation have allowed Italian hospitals to legally request shipments of Celginase, a low-cost cancer medicine shown to be substandard, even when better alternatives have been available.
Neither Italy's drugs regulator nor the country's ministry of health are responsible for checking the quality, efficacy or safety of this drug before allowing it into Italian hospitals. Nor does this fall within the remit of the European Medicines Agency (EMA).
Hundreds of vials of the drug have arrived from India over the last seven years. It is unknown how many cancer patients could have experienced adverse side effects or lower chances of remission as a result. Many vials still sit on hospital shelves today.
Celginase is a brand of asparaginase, a drug used to treat acute lymphoblastic leukemia, the most common form of childhood cancer. Celginase is made and approved in India, and costs a tiny fraction of the price of the “gold-standard” brand: as little as €13 a vial compared with about €2,500. Published academic studies have found it did not meet minimum manufacturing standards or consistently reach the clinical activity threshold to treat cancer.
In January, TBIJ and STAT revealed that poor-quality brands of asparaginase, including celginase, have been shipped to more than 90 countries since 2016, putting an estimated 70,000 children around the world at risk.
In 2018, an apparent nationwide shortage of Oncaspar, the “gold-standard” asparaginase, led to children in Italy receiving another brand, Aspatero, which was later found to be substandard. (The hospital concerned said its request to import Aspatero was authorized by the Italian drugs regulator, and that not doing this would have “reduced the chances of children's recovery.” The drugs regulator confirmed it had authorized the import.)
But TBIJ and POLITICO can now reveal that Celginase has been purchased by Italian hospitals even when the gold-standard product has been available – in some cases as recently as this year. Further records show that Celginase was also imported by seven Italian regional health departments, suggesting that the actual number of hospitals using the drug could be far higher.
Informed consent paperwork does not detail the brand of the drug that patients will be given nor the countries in which it is approved. Patients are being left in the dark.
Click on the button to load content from flo.uri.sh.
Lack of liability
There are several different types of asparaginase. “Native” asparaginase is made from Escherichia coli (E. coli). Turning this bacterium into medicine is complicated and even good-quality native asparaginase can cause side effects, including severe allergic reactions.
For this reason, doctors prefer to use modified versions of asparaginase. These are less likely to cause allergic reactions but are much more expensive up front.
Although Celginase and Oncaspar are both asparaginases, they are different: Celginase is native, Oncaspar is modified. Oncaspar has been approved for use in Europe since 2016 and is the brand of asparaginase recommended as first-choice treatment for acute lymphoblastic leukaemia.
In Italy, if a drug is in shortage, doctors and pharmacists can ask the Italian drugs regulator to greenlight the import of that same drug from another country.
A different law permits a drug that is not approved in the EU but is elsewhere in the world – such as Celginase – to be imported for an individual named patient if a doctor deems there is “no valid alternative therapy available”. As Celginase and Oncaspar are not exact equivalents, doctors have been able to request Celginase imports even if Oncaspar is available.
Crucially, this process bypasses the country's drugs regulator. In Italy, permission is instead given by the ministry of health's customs agency, which is required only to check that the paperwork has been correctly filled out by the hospital and the drug arriving is the one requested.
It is not required by law to check the quality of imported drugs nor ask for data from the overseas regulators that approved them.
Instead, the responsibility for these drugs lies with the individual doctor who requests them. The Italian drugs regulatory agency said that doctors should use qualified and reliable import intermediaries who can guarantee the quality and safety of the drug, particularly with suppliers in non-EU countries, and that doctors should get informed consent by giving the patient complete, transparent and exhaustive information on the drug.
Through freedom of information requests to the Italian customs agency of the ministry of health, TBIJ and POLITICO found that at least 16 hospitals, including the Istituto Nazionale Tumori in Milan and San Camillo Forlanini in Rome, imported hundreds of vials of Celginase into the country over a seven-year period. (The Istituto Nazionale Tumori said that shortages required them to source Celginase. It added that the patients who received the drug are either responding to it or are in remission, and that the Italian drugs regulator has never provided information showing that Celginase could be harmful. San Camillo Forlanini did not respond to a request for comment.)
Attilio Guarini, a doctor who treats patients with acute lymphoblastic leukaemia at Policlinico di Bari hospital, says there is no clinical guidance recommending doctors turn to a native asparaginase – such as Celginase – if Oncaspar is available.
The Italian drugs regulator told TBIJ that it denies any responsibility for the “efficacy and safety” of medicines imported from non-EU countries that are not authorized in accordance with EU regulations.
The European Medicines Agency (EMA) also has no power over imports of unapproved drugs from non-EU countries, according to its former head Guido Rasi.
Most of the Celginase imported into Italy from India came via Switzerland. The Swiss customs agency said it does not have specific data on the products we asked about. It did not know if Indian asparaginase imported via Switzerland may have gone to any other EU countries besides Italy, but it is understood that Celginase was not used in Swiss hospitals.
The EU issue
In certain situations, such as drug shortages, it is vital for European countries to be able to import medicines that may not be approved in the EU.
In 2001, the EU published new bloc-wide legislation allowing medicines that “fulfil special needs” to be excluded from the standard European rules around quality, safety, efficacy and manufacturing. But only under strict conditions.
Any such medicines (which may include those not approved in the EU) need to be formulated according to the specifications of an authorized healthcare professional – such as a doctor or a pharmacist – and given to an individual patient under this worker's "direct personal responsibility" .
Each country has incorporated this into its own national law in different ways.
In Italy, the prescribing doctor must justify the exceptional use, which is then checked by the customs agency of the ministry of health. In Spain, the hospital or health department must request access to the unlicensed drug from the regulator. In Ireland, the doctor issues the prescription and the wholesaler or manufacturer is required to notify the regulator of the import.
And while the EU law was meant to be applied to individual patients, in practice this is not always the case. In Germany and Italy, for instance, there are provisions for orders large enough to temporarily stock a hospital pharmacy. In Ireland, the law has been used so extensively that in 2020 more than 1.5 million packs of the 50 most popular drugs were brought into the country under the rules.
“If products for commercial sale are routinely entering the EU as unlicensed medicines and they've been supplied under the named-patient […] regime then that's an issue,” says Grant Castle, partner at Covington law firm.
He said these rules allow patients to access medicines where there is a bona fide medical need and are “not a suitable basis” to import medicines for other reasons. “If that's happening, it's a loophole.”
While it is down to member states to put rules in place for named-patient supplies of unlicensed medicines, Castle added, national rules “generally do not provide the same safeguards”.
Gilles Vassal, a pediatric oncologist and board member of the European Society for Pediatric Oncology, agreed. When addressing shortages by importing medicines unauthorized in the EU, he asks: “What are the measures set up at [the national] level to control the quality of the medicine they are importing?”
The Italian drugs regulator said that in February 2023, it exchanged information with WHO on data around a potential lack of efficacy of the Indian-sourced drug Aspatero provided by the study highlighted in TBIJ's first story. It added that there appears to be no evidence from the WHO or national regulatory authorities of low-quality, dangerous or ineffective asparaginases imported from India.
However, a European Commission spokesperson said that the Italian drugs regulator and other Italian authorities have been investigating this issue, which involves some specific Italian hospitals. A WHO spokesperson said it had contacted the countries reported in TBIJ's original investigation into asparaginase, but that no “actionable information” had been received.
Changes to the EU law are also in the works to attempt to address some of these concerns. A planned revision of the bloc's pharmaceutical legislation would include a line stipulating that countries "shall encourage" doctors and patients to report data on the safety of the use of unlicensed medicines to their own regulator.
It would also oblige drugmakers to flag any potential shortages six months in advance – up from two months – and it could require companies or wholesalers to keep larger stockpiles.
In Italy, the customs agency has responded to TBIJ's inquiries by working to modify its forms so doctors must give more detail about clinical reasons for the import. “We realized that there are loopholes in the system, so we are now working to fix them,” said Ulrico Angeloni, one of the agency's coordinators.
Doctors that TBIJ and POLITICO spoke to would like to see national measures to verify the quality of unlicensed drugs accessed via these loopholes, as well as more transparency on their use, effectiveness and safety.
For now, the paradox remains that while Europe's medicines are among the most highly regulated in the world, drugs that are shipped in from abroad can fail to meet even minimum standards.
The manufacturer of Celginase did not respond to a request for comment.
Reporters: Laura Margottini and Rosa Furneaux
Additional reporting: Maria Cristina Fraddosio
Illustration: Evangeline Gallagher
Deputy editor: Chrissie Giles
Editor: Meirion Jones
Production editor: Alex Hess
Fact checkers: Alice Milliken and Meriem Mahdhi
Impact Producer: Paul Eccles
This article is part of our Global Health project, which has a number of funders including the Bill & Melinda Gates Foundation. None of our funders have any influence over the Bureau's editorial decisions or output.
Related news: Quality of drugs, the report of the Food & Drug Administration
Fiscal Year 2022 Report on the State of Pharmaceutical Quality