
It is up to AIFA and the Technical-Scientific Commission to define innovation, evaluate it and confer the status of innovative medicinal product which presupposes the evaluation of three basic elements: therapeutic need, added therapeutic value and robustness of the scientific tests submitted by the company to support of the request for innovation. AIFA has established that the evaluation of this attribute must take place through a single model for all drugs, but allows, if necessary, the use 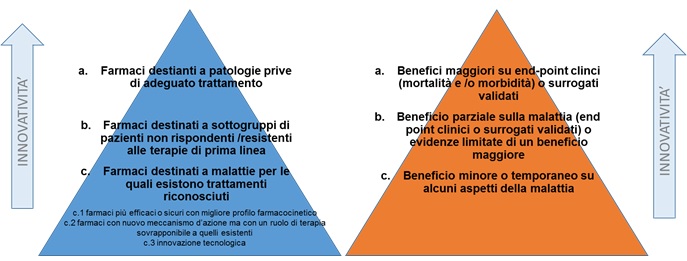 of further specific indicators.
of further specific indicators.
Criteria for the classification of innovative drugs and innovative oncological drugs
With the determines no. 1535/2017 AIFA has identified the criteria for the classification of innovative drugs and innovative oncological drugs pursuant to article 1, paragraph 402 of the law of 11 December 2016, n. 232.
PDF Attachment 2 – Application form for recognition of innovativeness [0.39 Mb] >
The Agency makes available the evaluation reports for the recognition of innovativeness, for therapeutic indication, in compliance with the provisions of the AIFA Resolution n.1535/2017.
Evaluation report of innovativeness by therapeutic indication (13/09/2019) [0.02 Mb] >
AIFA publishes the updated list of medicinal products which, in the opinion of the Technical-Scientific Commission, possess the requisite of therapeutic/important innovation and/or potential/conditioned therapeutic innovation, pursuant to article 10, paragraph 2 of Law no. 189/2012, as defined by article 1 paragraph 1 of the State-Regions agreement of 18 November 2010 (Rep.Atti n.197/CSR).
PDF List of innovative drugs (Law 189/2012) (10/09/2019) [0.24 Mb] >






