
THE governments of the last twenty years, in agreement with the largest drug multinationals, have well understood that encouraging the progression of a disease, rather than seeking a cure, is an enormous source of income. This is the case of Hepatitis C, a liver disease caused by the HCV virus which first causes liver malfunction, then scarring of the liver tissue (cirrhosis), and later the development of liver cancer.
governments of the last twenty years, in agreement with the largest drug multinationals, have well understood that encouraging the progression of a disease, rather than seeking a cure, is an enormous source of income. This is the case of Hepatitis C, a liver disease caused by the HCV virus which first causes liver malfunction, then scarring of the liver tissue (cirrhosis), and later the development of liver cancer.
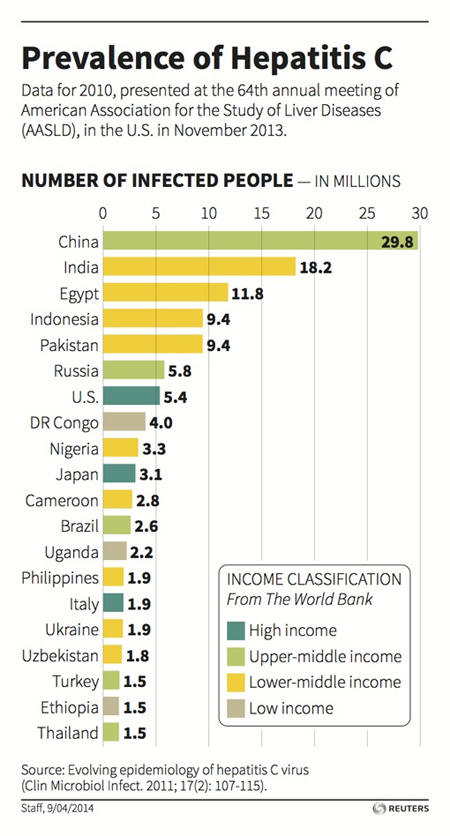
The disease, often silent for years after the infection, is so widespread due to the infections contracted, in the past decades, with the use of infected blood and material that has not been sterilized correctly. Our country has about 800,000 people affected by HCV.
The treatment of hepatitis C has undergone considerable progress. Until recently, the success rate of drugs was only around 50%, due to the severe side effects of the same. In recent years, fortunately, various combinations of drugs have been used, capable of acting on different mechanisms.
For almost three years, a new molecule has been marketed, a new antiviral drug that directly prevents the replication of the HCV virus, by inhibiting the enzyme RNA-dependent RNA polymerase (NS5B), the name of whose active substance is Sofosbuvir; the commercial name is, however, Sovaldi.
 The substantial difference between Sofosbuvir and other drugs used to eliminate the hepatitis C virus (such as Peg-IFN and ribavirin) is that this new drug acts directly against the virus, blocking its replication process. Interferon, on the other hand, stimulates the immune system's response against the virus and ribavirin indirectly interferes with viral replication. The combined intake of these drugs increases the possibility of definitive eradication of the virus. Sofosbuvir can only be taken together with ribavirin and/or pegylated interferon, starting from the first day of treatment.
The substantial difference between Sofosbuvir and other drugs used to eliminate the hepatitis C virus (such as Peg-IFN and ribavirin) is that this new drug acts directly against the virus, blocking its replication process. Interferon, on the other hand, stimulates the immune system's response against the virus and ribavirin indirectly interferes with viral replication. The combined intake of these drugs increases the possibility of definitive eradication of the virus. Sofosbuvir can only be taken together with ribavirin and/or pegylated interferon, starting from the first day of treatment.
Sofosbuvir (Sovaldi) was discovered by Pharmasset and then acquired for commercialization by Gilead Sciences. In Italy, Sovaldi has been marketed since March 2013 and can be prescribed by centers authorized by the Italian Medicines Agency (therefore paid by the NHS), only for patients with chronic hepatitis C in advanced stage of disease, for patients with cirrhosis of the liver or for patients who are candidates for liver transplantation.
THE patients with early stage disease can be treated with sofosbuvir only at their own expense (35 thousand euros);therefore only the rich will be able to cure themselves. In fact, in the past months, the Public Prosecutor's Office of Turin has opened a file against unknown persons for omission of treatment and culpable injuries, in relation to the issues of sofosbuvir and its costs for the public health system.
patients with early stage disease can be treated with sofosbuvir only at their own expense (35 thousand euros);therefore only the rich will be able to cure themselves. In fact, in the past months, the Public Prosecutor's Office of Turin has opened a file against unknown persons for omission of treatment and culpable injuries, in relation to the issues of sofosbuvir and its costs for the public health system.
The most disputed data sees the enormous difference in the price of the drug between our country (35 thousand euros) and India, a country where the entire therapeutic cycle does not exceed i One thousand Euro.
At the basis of the high costs there would be experimentation and patents. As Luca Pani, president of Aifa, already stated last year, «The value of a drug is never that of its production as such but the mechanisms that lead to the definition of the final price must be understandable, taking into account, for example, investments in clinical development, trials and even reasonable patent protection. What seems unclear is how the manufacturing company determined the price of Sofosbuvir, which, according to AIFA estimates, is in any case higher than expected, even considering development and production costs".
 Reason why the "Journeys of Hope" to India continue. Why? Last January, the Delhi patent office rejected the manufacturer's request for registration, deeming its molecule, i.e. sofosbuvir (the active principle of Sovaldi), not innovative enough.
Reason why the "Journeys of Hope" to India continue. Why? Last January, the Delhi patent office rejected the manufacturer's request for registration, deeming its molecule, i.e. sofosbuvir (the active principle of Sovaldi), not innovative enough.
Fighting the so-called mechanism ofevergreening (i.e. "the so-called "Always Green Patent", i.e. a market strategy of pharmaceutical companies to keep the patent on a product as much as possible by making small changes, such as adding an excipient) it is a vital issue for the country. It's about defending the right to health for its 1.2 billion inhabitants, half of whom live below the poverty line. Backed into a corner, Gilead has licensed generic drug companies to manufacture the pill and sell it for $1 in 91 developing countries, including India.
Italy, in compliance with international laws on patent protection, cannot buy Sovaldi from Indian generic companies. India has managed, once again, to confirm itself 'the low-cost pharmacy of the globe', being the world's leading supplier of generic drugs for the treatment of cancer, tuberculosis and AIDS. In fact, his battle against the caste of the pharmaceutical industries has been going on for years.
In Italy, 70 percent of patients cannot be treated at their own expense given the excessive cost of therapy; so why limit the use of sofosbuvir only in the advanced stages of the disease? Early treatment would mean eradicating the virus before it irreversibly damages the liver.
In Italy, therefore, do we want to make health or, rather, disease contagious?
Of Enrico Alagna | January 14, 2016 | Il Fatto Quotidiano.it
Related news: Chronic hepatitis, a contact center at the Federico II Polyclinic to facilitate communication between doctors and patients






