
Definition of orphan drug status
economy and finance – 11 July 2021
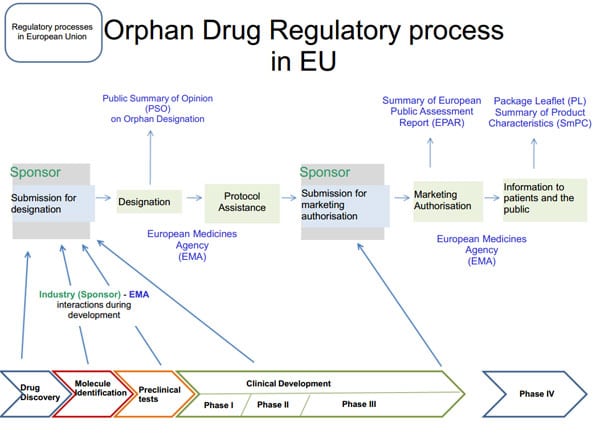 Orphan drug status gives companies researching treatments for rare diseases a seven-year fee reduction window and the exclusive right to develop treatments for specific diseases. Orphan drug status can be granted to new drugs, approved drugs, or drugs already on the market. However, if the drug is approved, the sponsor must present a reasonable hypothesis as to how the drug is clinically superior to previous or undeveloped drugs.
Orphan drug status gives companies researching treatments for rare diseases a seven-year fee reduction window and the exclusive right to develop treatments for specific diseases. Orphan drug status can be granted to new drugs, approved drugs, or drugs already on the market. However, if the drug is approved, the sponsor must present a reasonable hypothesis as to how the drug is clinically superior to previous or undeveloped drugs.
Key points
- Orphan drug status gives the company exclusive marketing and development rights and other benefits to recoup the cost of developing drugs for rare diseases.
- The Orphan Drug Act aims to encourage companies to develop medicines for rare diseases.
- The FDA can revoke the orphan drug status.
- However, compared to expensive and rare diseases and conditions, pharmaceutical companies prefer to treat cheaper diseases and conditions.
Understand the status of orphan drugs
In 1982, the United States Food and Drug Administration (FDA) recognizes that pharmaceutical companies lack the motivation to develop treatments for rare diseases. Based on this recognition, the Orphan Drug Act of 1983 was born. The plan aims to target diseases affecting fewer than 200,000 people in the United States.
The Office of Orphan Product Development (OOPD) encourages companies to exercise their rights under the Orphan Drugs Act of 1983. The OOPD develops and awards grants for companies, biologists, clinicians and researchers who wish to develop products and drugs to the treatment of these rare diseases. The law refers to these groups as sponsors.
The Orphan Drug Act has been revised many times to include products other than drugs, such as biological products, medical devices, and medical foods (mainly prenatal foods).
Items of special attention
 As we all know, pharmaceutical companies are businesses first and therapists second. Pharmaceutical companies spend billions of dollars each year on R&D For example, Pfizer (PFE) will have revenues of $41.908 billion in 2020. R&D spending that year was approximately $9.4 billion. This equates to 22.4% of R&D revenues.
As we all know, pharmaceutical companies are businesses first and therapists second. Pharmaceutical companies spend billions of dollars each year on R&D For example, Pfizer (PFE) will have revenues of $41.908 billion in 2020. R&D spending that year was approximately $9.4 billion. This equates to 22.4% of R&D revenues.
If the company fails to obtain a patent, developing new drugs is also a risky business. There is also stiff competition from counterfeit medicines and generics or similar medicines. Many companies go to places where it's relatively easy to make money.
The pros and cons of orphan drugs
In addition to research into exclusive rights and tax credits, the FDA will also provide technical assistance for orphan drug applications, approvals that can shorten the waiting period, and discounts on registration fees. The state also plans 25% Tax credit As for the cost of clinical trials.
Orphan drug status is not designed to allow sponsors to recover all drug development costs, but to reduce costs and simplify regulatory mechanisms. The FDA can easily revoke the orphan drug designation. Common reasons include: misrepresentations or missing information in the designation application or the FDA believes the disease or condition will affect more than 200,000 people in the future.
Developing drugs to treat a large number of diseases around the world is a business that can bring enormous wealth. However, in the pharmaceutical industry, the greatest wealth is often achieved through the development of standard drugs for the treatment of common diseases. From a commercial point of view, having a large market can ensure that companies can quickly recover development costs and obtain maximum benefits.
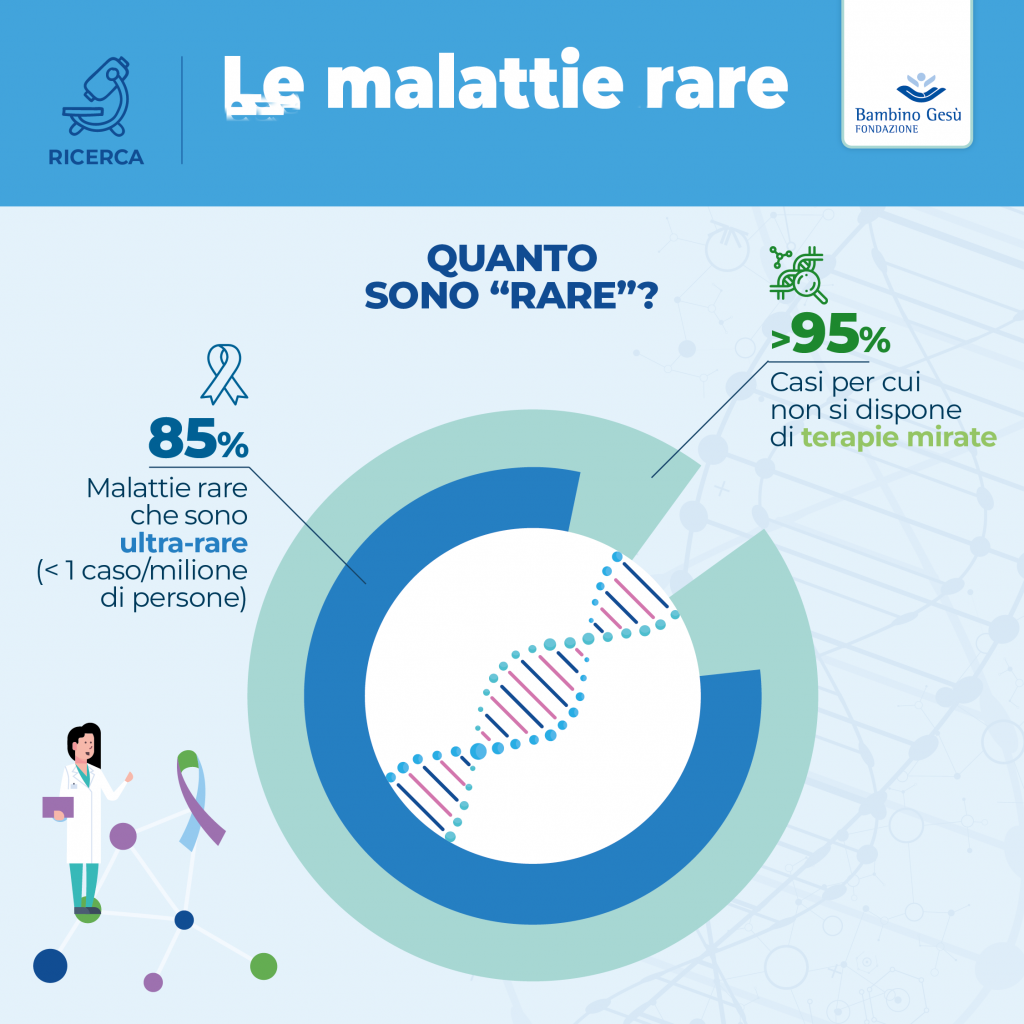
Note: In Europe, a disease is considered rare when it affects no more than 5 people for every 10,000 inhabitants.
Related news: AIFA. Orphan drugs
Orphan drug lists 31 December 2020 [0.14 Mb]
Government. Rare diseases. Orphan drugs (regulation)
Rare diseases and orphan drugs, presented the 4th OSSFOR Report
Orphan drugs have become a business by dint of incentives for companies





