Yes to the reimbursement of off-label drugs. This is the sentence with which the Court of Justice last November 21 put an end to the long European dispute that opened after the decision of the Antitrust to fine Novartis and Roche in 2014 with a fine of over 180 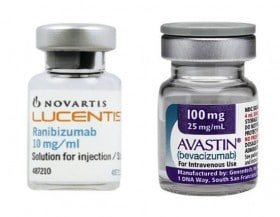
It all started when the Council of State asked the Court of Justice the question of the legitimacy of the AIFA decision, in June 2014, to provide for the reimbursement of Avastin in off-label mode, for reasons exclusively related to cost containment. The Court confirmed the full autonomy of each Member State to decide on questions relating to the reimbursement of medicines on its national territory. Furthermore, the legitimacy of the repackaging of the drug in question by hospital and community pharmacies was reaffirmed, thus reaffirming that, in this specific case, we are not dealing with masterful preparations.
Finally, it is confirmed that the exclusive competence of the EMA concerns the examination of applications for MA and, in this case, the repackaging activity does not require obtaining a new MA and therefore cannot harm any exclusive competence of the European Agency of the drug.
Related news: Avastin-Lucentis, dismissed the appeal of Novartis. Now Minister Grillo will (finally) have to act
