One year after the introduction of the #Code of Ethics have been pre-evaluated in the Conference Evaluation System #SVC 2,516 training events: only the 3% was considered non-compliant with our requirements.
Our president, Massimiliano Boggetti, underlines that: "we continue to play a proactive role in the fight against corruption in healthcare, the balance is positive, now it is important to relaunch the country with scientific collaboration projects that enhance our excellence".
Medical Devices: The 97% of educational events complies with the industry code of ethics
First positive balance for the new one Ethical code of Confindustria medical devices, which introduced in July ofLast year the Conference Evaluation System (SVC) to analyze compliance with the "sobriety" requirements in training events such as congresses, workshops or medical-scientific seminars. According to the data  communicated today by the business association, out of 2,516 initiatives only the 3% was found not to comply with the new rules.
communicated today by the business association, out of 2,516 initiatives only the 3% was found not to comply with the new rules.
The criteria of sobriety
Among the criteria of sobriety, the prohibition of choosing 5-star or higher category hotels, seaside or mountain tourist resorts in high season, first class travel, including extra or recreational activities, accompanying persons at the expense of the organization or extending the trip at the expense of the organization.
Events
There were 682 third parties who entered training events in the Svc platform managed by Confindustria Dm. In 83% of the cases these are CME events from 92 therapeutic areas. In most cases these are events aimed at general medicine (593), internal medicine (538) and cardiology-cardiovascular (525). Followed by diabetology (321), radiology (279), molecular biology (258), laboratory medicine (94).
The events analyzed are above all congresses, conventions or conferences that saw the participation of 267,678 people (85%), then theoretical courses with 23,425 participants (7%), finally theoretical-practical courses with 22,152 participants (7%). The 84% of the events had multiple sponsors, while the 16% was a single sponsor.
The comment of Confindustria medical devices
"The balance one year after the introduction of the new code of ethics for medical device companies - comments Massimiliano Boggetti, president of Confindustria medical devices - has made it possible to map a process that was essentially already organized in terms of sobriety and responsibility on the part of our companies. We have contributed to intercepting non-compliant events, even if they are a small number compared to the total, introducing principles of greater transparency which have proved to be successful. All this - continues Boggetti - will allow us not only to continue to play a proactive role in the fight against corruption in healthcare, but above all to give our contribution to encourage the development of biomedical research in Italy and investments in the sector, establishing a virtuous circle for the country, which stimulates collaboration between doctors, industry, hospitals, universities, research and technology transfer centers in a transparent way".
Indirect sponsorship
All the companies registered in the association have joined the new Code of Ethics, also undertaking to introduce indirect sponsorship, i.e. the provision of their contribution for the training of healthcare professionals to third parties, such as providers, healthcare facilities or scientific societies, without intervening in the choice of professionals who benefit from it, the scientific program and the speakers.
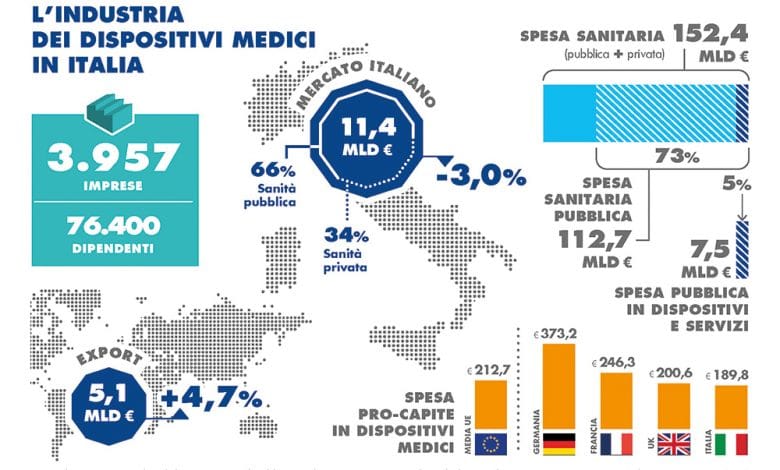
Related news: the DM sector in numbers






