The Italian Medicines Agency has published the twelfth Pharmacovigilance Report on anti-COVID-19 vaccines. The data collected and analyzed concern the reports of suspected adverse reactions recorded in the 
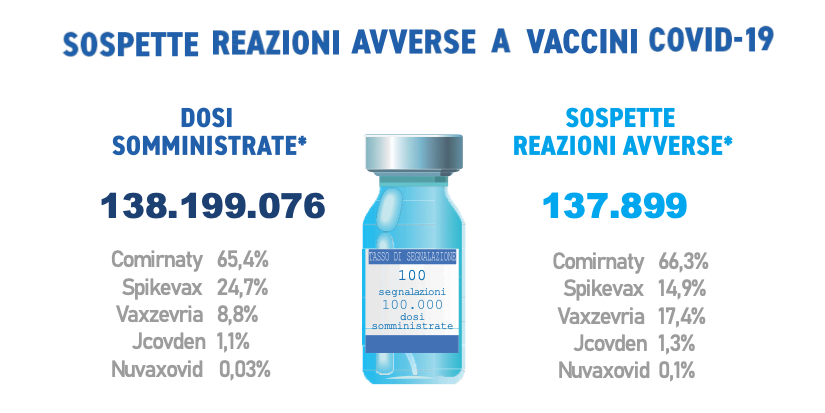
They were received during the period in question 137,899 reports out of a total of 138,199,076 doses administered (reporting rate of 100 per 100,000 doses), of which the81,8% referring to non-serious eventssuch as injection site pain, fever, asthenia/fatigue, muscle aches.
In the second quarter of 2022, 1st dose reporting rates remain higher than subsequent doses and are lowest after the 4th dose for all vaccines.
The serious reports correspond to 18.1% of the total, with a rate of 18 serious events every 100,000 doses administered, in line with the previous Reports. It should be remembered that the seriousness of the reports is defined on the basis of standardized criteria which do not always coincide with the real clinical seriousness of the event.
The adverse reaction occurred in most cases (approximately 71%) on the same day of vaccination or the following day and only more rarely after 48 hours, regardless of the vaccine, the
Comirnaty is the currently most widely used vaccine (65.4%), followed by Spikevax (24.7%), Vaxzevria (8.8%), Jcovden (ex-COVID-19 Janssen vaccine) (1.1%) and Nuvaxovid (0.03%), in use since 28 February 2022. In line with the previous Reports, the distribution of reports by type of vaccine follows that of administrations, with the exception of Vaxzevria and Spikevax which appear to be reversed in this trend (Comirnaty 66.3%, Vaxzevria 17.4% , Spikevax 14.9%, Jcovden 1.3%, Nuvaxovid 0.1%).
For all five vaccines, the most commonly reported adverse events are fever, headache, muscle/joint pain, chills, gastrointestinal discomfort, vegetative reactions, fatigue, local reaction, or pain at the injection site.
In the 5-11 year age group, as of 06/26/2022 a total of 471 reports had been entered (about 0.5% of the total) for the Comirnaty vaccine, the only one currently authorized for this age group, with a reporting rate about 18 cases per 100,000 doses. Approximately 95% of these reports is attributed to the 1st dose and approximately 5% to the 2nd. The most frequently reported adverse events, regardless of severity and causality, were injection site pain, headache, fever, and fatigue.
The data contained in this Periodic Report are consistent with those published to date and in line with the safety information already discussed at European level.
AIFA – Comunicato pubblicato il: 27 luglio 2022
Twelfth AIFA Report on the surveillance of anti-Covid 19 vaccines
Related news: How to report an adverse reaction
Monitoraggio Covid-19, report 18 – 24 luglio 2022