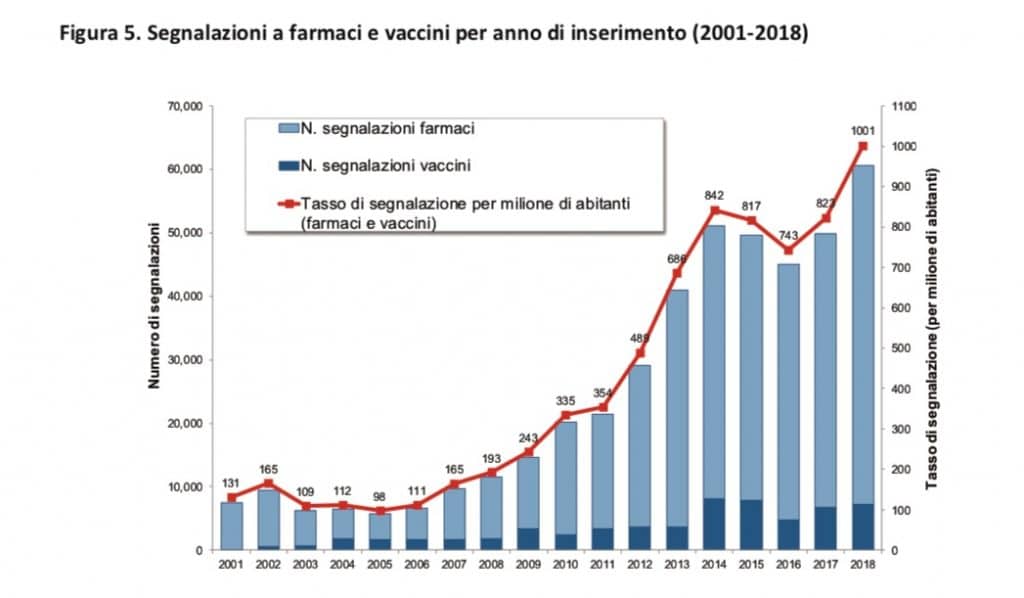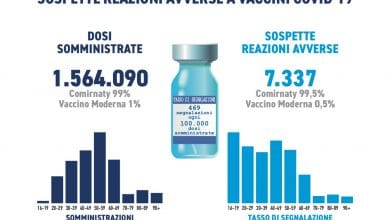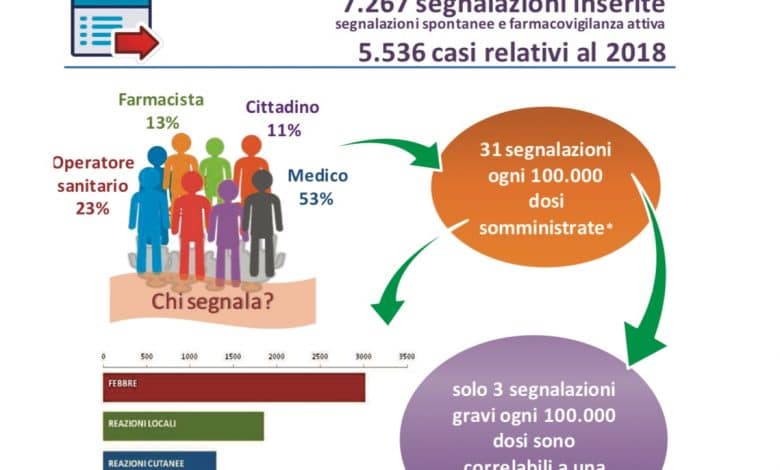
The Vaccine Report summarizes the post-marketing surveillance activities on vaccines conducted in Italy in 2018. Compared to previous reports, this year it was possible to use, for the calculation of the reporting rates (ratio between the number of reports and the data of exposure), the doses actually administered at national level, provided by the Ministry of Health and by the Departments of Prevention of the Regions and Autonomous Provinces.
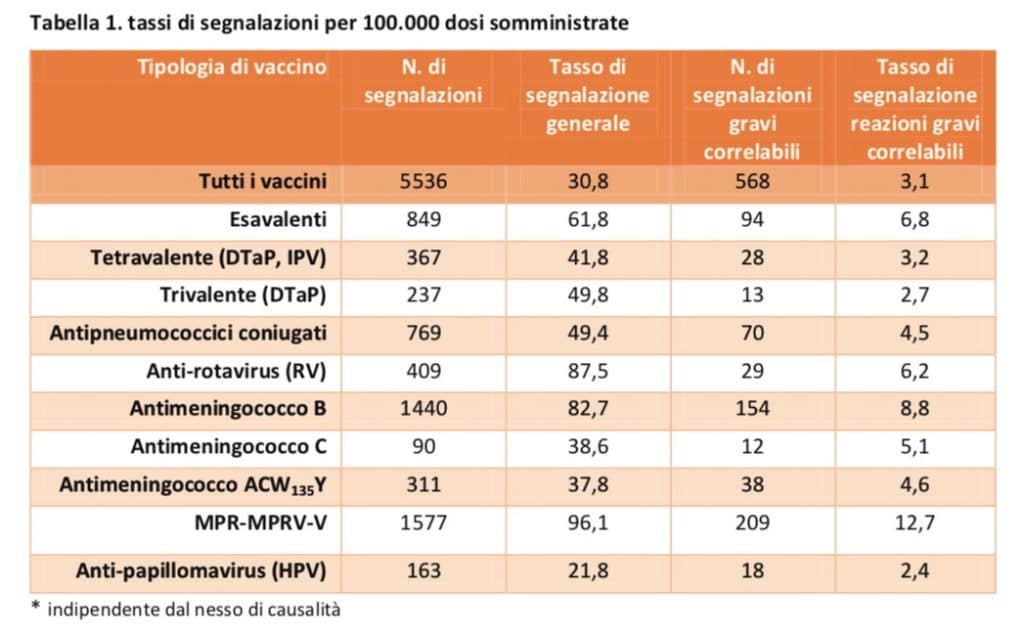 This allowed the calculation of overall reporting rates and correlated serious adverse reactions rates per dose administered nationwide. Overall, out of a total of about 18 million doses administered in Italy in 2018 for all types of vaccine, 31 reports were made for every 100,000 doses, which corresponds to about 12 reports for every 100,000 inhabitants.
This allowed the calculation of overall reporting rates and correlated serious adverse reactions rates per dose administered nationwide. Overall, out of a total of about 18 million doses administered in Italy in 2018 for all types of vaccine, 31 reports were made for every 100,000 doses, which corresponds to about 12 reports for every 100,000 inhabitants.
The reporting frequency of correlated serious adverse reactions is 3 events per 100,000 doses. The correlated reactions reported are all known and therefore already reported in the product information of the vaccines authorized in Italy.
The growing trend in the number of suspected adverse reactions is indicative of an increasing attention to vaccine surveillance by both health professionals and citizens. From the analysis of the national data, no information emerged that could influence the benefit-risk ratio for the various types of vaccine currently used, thus confirming their safety.
In addition to the in-depth analysis of suspected adverse reactions for each type of vaccine, as every year, a focus was dedicated to anti-flu vaccines and some insights which, in this edition, concern international vaccinations for travelers and the vaccinations recommended in pregnancy.
AIFA Published on: 30 July 2019