
Awarded to American James P. Allison and Japanese Tasuku Honjo “for their discovery of cancer therapy  through the inhibition of negative immune regulation”, which unlocks the brakes that some tumors are able to impose on the immune system.
through the inhibition of negative immune regulation”, which unlocks the brakes that some tumors are able to impose on the immune system.
The Sciences – October 1, 2018
The Nobel Assembly at the Karolinska Institut in Stockholm has awarded this year's Nobel Prize in Medicine or Physiology to James P. Allison and Tasuku Honjo. The two researchers were awarded for the discovery of a cancer therapy through the inhibition of the negative regulation of the immune response, i.e. the deactivation of the "brakes" that some types of tumor manage to impose on the immune system
Unblock the immune system to fight cancer
By unblocking the brakes that some types of tumors manage to impose on the immune system with targeted antibodies, it is possible to obtain a clinical response in at least a part of patients suffering from various forms of cancer. The demonstration comes from a series of clinical studies that have also identified a biological marker that allows the efficacy of this type of therapy to be predicted with good reliability.
The sciences
Promising clinical responses in patients with various types of cancer have been obtained thanks to therapies that aim to awaken the immune system's response to tumors. This was reported by five new studies published in "Nature" which expand the number of known tumors that respond to immunotherapies and increase knowledge on the characteristics of tumors that allow us to anticipate the level of response of tumors to therapies.
These new therapies aim in particular at unlocking molecular brakes – also called checkpoints or checkpoints immune systems – which normally prevent the immune system from becoming too destructive by attacking healthy cells in the body (as occurs in autoimmune diseases). However, some tumors have the ability to co-opt these molecular brakes by weakening the immune response against the tumor's degenerate cells.
 T lymphocytes attacking a cancer cell. (© Photo Quest Ltd/ /Science Photo Library/Corbis)
T lymphocytes attacking a cancer cell. (© Photo Quest Ltd/ /Science Photo Library/Corbis)
These inhibitory pathways are triggered by the binding of particular molecules, the ligands, to two receptors, called CTLA-4 and PD-1, present on the surface of T lymphocytes and other cells of the immune system; in the case of the PD-1 receptor this binding even leads to the direct death of the cell.
The new immunological therapies are based precisely on the administration of antibodies capable of blocking the binding site of the CTLA-4 (ipilimumab antibody) and PD-1 (pembrolizumab and nivolumab) receptor binding sites, so as to prevent them from being reached by the ligands that would activate them. But until recently, the effectiveness of these treatments had only been documented in cases of melanoma and renal cell carcinoma. Furthermore, neither the exact cellular events triggered by antibody binding nor their precise antigenic targets, i.e. the molecular structures of the immune cells to which they bind, were fully understood.
Thomas Powles and colleagues and from Roy S. Herbst and colleagues broaden the applicability and safety of the use of antibodies that block immune inhibitory pathways in patients with urothelial bladder cancer, non-small cell lung cancer, renal cell carcinoma, squamous cell carcinoma of the head and neck, respectively neck, colorectal and stomach cancers, although lasting results of the therapy were found only on a subset of the treated subjects.
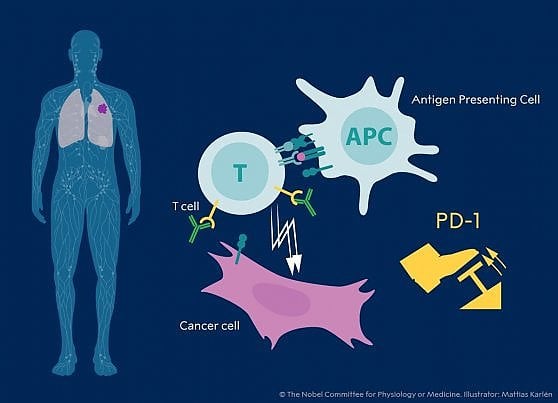 To this subgroup - both Herbst and colleagues showed and, in another study, Paul C. Tumeh and colleagues – belong to subjects in which the immune system had had time to recognize the initial development of the tumor before it was able to block the immune response. Furthermore, Tumeh and colleagues have also demonstrated that by analyzing CD8+ T lymphocytes it is possible to detect the presence of a biological marker that signals whether this has occurred or not. This marker therefore represents a useful predictor of the efficacy of immunological therapy.
To this subgroup - both Herbst and colleagues showed and, in another study, Paul C. Tumeh and colleagues – belong to subjects in which the immune system had had time to recognize the initial development of the tumor before it was able to block the immune response. Furthermore, Tumeh and colleagues have also demonstrated that by analyzing CD8+ T lymphocytes it is possible to detect the presence of a biological marker that signals whether this has occurred or not. This marker therefore represents a useful predictor of the efficacy of immunological therapy.
In the last two articles, Mahesh Yadav and colleagues And Matthew M. Gubin and colleagues they focused on the characteristics of the antigens that allow the inhibition of the immune response to be triggered and of the tumor antigens that could in any case trigger it because they are highly immunogenic. In this way they discovered that they are encoded by genes whose mutation hardly contributes to the development of cancer and confirmed the hypothesis, already advanced for some time, according to which changes in the level of immunogenicity of tumor cells can derive from "secondary" mutations. which, however, could in the future become a further objective of immunological therapies.
•THE MOTIVATION
The two scholars, reads the official motivation, "have understood that it is possible to stimulate the immune system to attack tumor cells, an absolutely new therapy mechanism in the fight against a type of disease that kills millions of people every year and which constitutes a of the gravest threats to human health". With this strategy, Stockholm continues, “this year's Nobel winners have discovered a completely innovative principle”. Immunotherapy has already been used in the fight against some cancers, both solid and blood, for several years, with promising results. For Giorgio Minotti, dean of the Faculty of Medicine and Surgery of the Biomedical Campus in Rome, “this discovery affects the health of many patients and has exceptional value. The award represents an accelerated investment in the future of this strategy. Given its costs, we must now also address the problem of sustainability". In addition to the price, some shadows concern the lack of response from some patients. “This happens without us fully understanding why. We still have a lot to study on this mechanism” explained Minotti.
Related news: Immunotherapy: the story of Letterio Visigalli, the European patient zero
CAR-T. First anticancer cell therapy
Tumor immunotherapy, ACC: "Collaboration with Nobel laureate James P. Allison is underway"





