
HERE IS THE LIST OF ITALIAN DOCTORS AND FOUNDATIONS, UNIVERSITIES FINANCED BY GLAXO-SMITH-KLINE IN 2015, 2016 AND 2017
SURPRISELY, THE HIGHER INSTITUTE OF HEALTH APPEARS ON THE LIST
IL CODACONS: TRANSPARENCY IS A MUST, WHAT IS THIS MONEY FOR?
The Codacons has decided to publish on its website, for obvious reasons of transparency of correct information to citizens, the  list of Italian doctors and foundations/universities/institutes financed by the pharmaceutical company Glaxo-Smith-Kline, as distributed by EFPIA. Furthermore, Codacons will forward this list to the Anac, following the complaint already presented for conflict of interest against the president of the Istituto Superiore di Sanità Gualtiero Ricciardi.
list of Italian doctors and foundations/universities/institutes financed by the pharmaceutical company Glaxo-Smith-Kline, as distributed by EFPIA. Furthermore, Codacons will forward this list to the Anac, following the complaint already presented for conflict of interest against the president of the Istituto Superiore di Sanità Gualtiero Ricciardi.
The EFPIA (European Federation of Pharmaceutical Industries and Associations) is in fact an association of pharmaceutical industries, with a direct representation of 33 national associations and 40 leading pharmaceutical companies, characterized by a strong emphasis on the transparency it requires of its affiliates. It is precisely to respond to this request that Glaxo-Smith-Kline has made public, among other things, the lists of Italian doctors who received funding in 2015/2016/2017 (as services, consultancy, various events) .
Despite the lack of detailed references to these loans, and for the most part we limit ourselves to generic references, the total donated by Glaxo-Smith-Kline appears to be growing: more than 11 million in 2015, more than 13 million in 2016, almost 15 million in 2017. now, the question is legitimate: what is this money for?
Among Universities, Foundations and Hospitals, it is surprising, in particular, to read the name of the Istituto Superiore di Sanità (125,660.00 euros in 2016, 93,940.00 euros in 2017 for "services and consultancy"): it appears at least inappropriate, in fact, that the technical-scientific body of the National Health Service is included in the list, and it would really be appropriate to explain the reasons for these transfers - disseminating the details - so as to increase transparency and knowledge on the point. Which, of course, could only contribute to the overall credibility of the National Health System.
Here are the reports cited:
GSK_IT_2015_EFPIA_HCPO_Disclosure_Report
GSK_IT_2016_EFPIA_HCPO_Disclosure_Report
GSK_IT_2017_EFPIA_HCPO_Disclosure_Report
Related news: GSK. ABAC – Anti-Corruption and Corruption Policy (pdf)
GSK extension. Code of practice for promotion and scientific engagement (pdf)
Ed: Codacons, as often happens, promotes initiatives likely to cause scandal in public opinion. In order to judge, however, it is necessary to know how things really are.
The publication of data relating to economic transactions for research, consultancy, congresses and training activities is envisaged by the Efpia Transparency Code, implemented in Italy with the update of the Farmindustria code of ethics. The data, published in full compliance with the Italian legislation on privacy, concern the individual healthcare professionals who have signed the consent and, in aggregate form, all the others (at least 70% of Italian doctors declared themselves in favor of making the fees public received from pharmaceutical companies). For each doctor who has given consent, the amount of the transfer disbursed, the name of the structure to which it belongs and the category in which the contribution falls (conferences, consultations, etc ...) are visible. To these data are added those relating to R&D grants, published in aggregate, as established by already existing regulations.
After corruption scandals, especially in China, GSK has given itself precise rules so as to avoid that 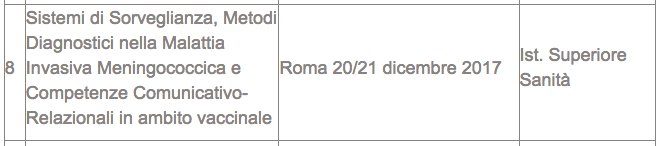 the existence of conflicts of interest may be perceived even if only potential between GSK itself, the Provider delivering the training initiative and the medical speakers and/or learners. GSK ensures to periodically update the details on the Independent Medical Education (IME) programs funded in Italy as part of its ongoing commitment to transparency and to make it clear to Healthcare Professionals its support for the constant updating and continuous training of the medical profession through the support for the best accredited educational initiatives, including the Istituto Superiore di Sanità (ISS).
the existence of conflicts of interest may be perceived even if only potential between GSK itself, the Provider delivering the training initiative and the medical speakers and/or learners. GSK ensures to periodically update the details on the Independent Medical Education (IME) programs funded in Italy as part of its ongoing commitment to transparency and to make it clear to Healthcare Professionals its support for the constant updating and continuous training of the medical profession through the support for the best accredited educational initiatives, including the Istituto Superiore di Sanità (ISS).
According to the reasons in favor of these transactions it is believed that the cooperation between pharmaceutical companies, regulatory bodies, healthcare professionals (HCPs), healthcare organizations (HCOs) and patients, is essential for the sustainable improvement of healthcare. As experts in their field, healthcare professionals provide first-hand scientific and medical knowledge and unique insights into patient care. This partnership is fundamental to research, to the advancement of medical science, helping to meet the diverse needs of patients and public health.
 Starting in 2016, EFPIA member companies have committed to disclose the transfers of value they make to HCPs and HCOs in relation to prescription medicines, on an individual (aggregated by exception only) basis. This includes, for example, sponsorship for travel and registration expenses to attend medical conventions, HCP consulting fees for speaker agreements or to chair meetings, and grants to HCOs. Transfers of value made to HCPs and HCOs for R&D-related activities are disclosed as an aggregated number. Disclosure information is published annually (covering the previous year) on a public platform. So does it too GSK extension in the published platform on your site
Starting in 2016, EFPIA member companies have committed to disclose the transfers of value they make to HCPs and HCOs in relation to prescription medicines, on an individual (aggregated by exception only) basis. This includes, for example, sponsorship for travel and registration expenses to attend medical conventions, HCP consulting fees for speaker agreements or to chair meetings, and grants to HCOs. Transfers of value made to HCPs and HCOs for R&D-related activities are disclosed as an aggregated number. Disclosure information is published annually (covering the previous year) on a public platform. So does it too GSK extension in the published platform on your site
In addition to the EFPIA code, Italian legislation regulates this type of "sponsorship" with art. 123 And 124 of Legislative Decree 219/06.
It follows that all of this is legitimate.
We can make the following considerations: healthcare professionals are indispensable to pharmaceutical companies because they can provide scientific knowledge to pharmacological research, at the same time pharmaceutical companies are becoming indispensable to doctors to whom they can provide resources, which the State does not give, for their research and medical training. It is positive that all this takes place with the utmost transparency to avoid incorrect operations. Then it always depends on the controls, which are what they are but which should be very careful and strict so that illegal activities are not hidden. Is all this ethically and politically correct? Everyone can give his answer based on his own conscience.
Obviously we are talking about research and training, scientific information comes later and makes use of research data and clinical work to be transmitted to health professionals.
We suggest that Codacons take care of the lobbying activity which has no regulation in Italy.
Unclear interests mean that rules cannot be approved that make the action of lobbyists transparent because otherwise the altars would fall. Lobbying can be correct, legitimate and clear, but the absence of a law prevents the voter from understanding what is really behind the amendment presented by the individual deputy, what interests and who drafted it; prevents knowing who pays and for what.
According to relationship drawn up in 2015 by the Corporate Europe Observatory (CEO), big pharma reported spending on lobbying activities, at European Community level, amounting to 40 million euros (in 2012, 8.3 million euros). An enormous economic power, through which it is able to influence the decision-making processes that take place in the community bodies. And in Italy?





