
In the production and R&D of drug companies, digital transformation has reached even higher levels than in other Made in Italy manufacturing sectors. On the other hand, there is ample room for improvement in the governance of data and their integrity, both at the level of infrastructures and systems and of processes and solutions
ZeroUno – 16 August 2018 – by Giuseppe Aliverti
More investments, more production, more exports: since 2010 the Italian pharmaceutical industry has entered an expansion phase which has led the sector to become a spearhead of our economy and to act as a driving force also for the various related sectors .
In the last seven years, the pharmaceutical sector has been first in Italy for growth in production (+13%), exports (+52%) and productivity (+19%). With a turnover of 30 billion euros (approximately 72% developed by exports), Italian production is worth 25% of the total of European production (figure 1). There are 64,000 direct employees (6,200 in Research and Development) and 66,000 in related industries. More than 50% of researchers are women, and about 3,000 more employees since 2014.
The escalation of Made in Italy Pharma has earned it a place of honor in Europe, and beyond: Italy is an international hub for the production of drugs and vaccines (second in Europe only to Germany) and aims to soon become a 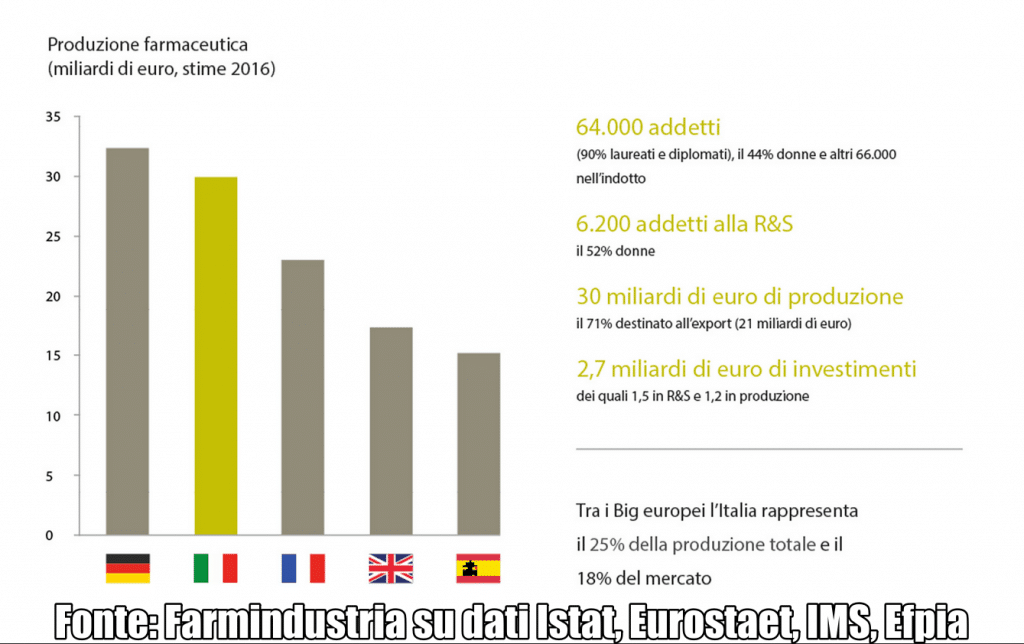 continental hub also for clinical studies.
continental hub also for clinical studies.
All results achieved above all thanks to the investment policy of many companies: not only conspicuous but also, for the most part, targeted and far-sighted. In particular, spending on digital technologies has reached 6.5 billion euros in recent years (estimates by Farmindustria, the Association of Pharmaceutical Companies). In 2016 alone, companies in the sector invested 2.7 billion euros (1.5 in R&D and 1.2 in production), with a growth of 450 million over the three-year period.
“The Italian pharmaceutical industry – explains Alberto Bartolini, Coordinator of the Supply Chain Area of Afi-Associazione Farmaceutici Industria – has been able to align itself in time to the change in perspective of the sector at a global level. The socio-demographic evolution of the countries with more advanced economies, on the one hand, with the prolongation of life expectancy, the  progress made by medicine on the other (for example in the treatment of so-called 'rare diseases', autoimmune diseases and food intolerances and in the fight against cancer) have led to an overall rethinking of drugs and their use. From the 'one drug fits all' paradigm we are moving on to the conception of tailor-made drugs: the formulation, production, dosage and methods of administration will be able to take into account the actual and measurable conditions of the patient. In a nutshell, the drug will become part of a virtuous and interactive circle of solutions integrated with diagnostic tools and health services. A revolution in which digital systems and solutions play an essential role, from the cloud toIoT, give her blockchain at 3D printers until'augmented reality. And in fact, there has been talk of Pharma 4.0 for some time now”.
progress made by medicine on the other (for example in the treatment of so-called 'rare diseases', autoimmune diseases and food intolerances and in the fight against cancer) have led to an overall rethinking of drugs and their use. From the 'one drug fits all' paradigm we are moving on to the conception of tailor-made drugs: the formulation, production, dosage and methods of administration will be able to take into account the actual and measurable conditions of the patient. In a nutshell, the drug will become part of a virtuous and interactive circle of solutions integrated with diagnostic tools and health services. A revolution in which digital systems and solutions play an essential role, from the cloud toIoT, give her blockchain at 3D printers until'augmented reality. And in fact, there has been talk of Pharma 4.0 for some time now”.
The centrality of the data
Today, the 80% of machinery and systems used in Italian companies is integrated or can be integrated in 4.0 mode. Over the next three years, according to a recent survey carried out by Farmindustria with Bain & Company, more than two-thirds of companies will continue to invest in digital technologies, with peaks of 88% in production and 71% in R&D.
“On the production front and, more generally, of supply chains – remarked Bartolini – the central issues are those of data integrity and governance, and will increasingly be so, due to the need to guarantee security, transparency and validation of information at every level and in every market, which involve numerous and complex compliance activities in companies, and the related inspections by Aifa, the Italian Medicines Agency”.
Today the production standards, the verification systems, the efficiency and the reliability of the supply chain of our companies are guaranteed, certified and recognized on a global level: otherwise we would not export the 72% of the production. But in terms of data transmission and management, there is still ample room for improvement both in terms of infrastructure and systems as well as processes and solutions: alongside widely computerized companies on this front as well, there are quite a few companies that they manage the enormous mass of data required with 'hybrid' procedures and approaches, where more or less extensive use is still made of paper documentation. The use of blockchain solutions would seem to be the best and fastest way to guarantee the integrity and confidentiality of data and prevent the risks of cyber attacks.
 "But there is an even more pressing push to digitize those data and processes that have so far been excluded and remained 'on paper' - warns Bartolini -: February 9, 2019 is almost upon us, when the serialization obligation, imposed by Directive 2011/62/EU. In other words, a unique serial number will be assigned, connected to individual production data (including product identification, expiry date and lot number, ed) in the form of a two-dimensional matrix barcode”.
"But there is an even more pressing push to digitize those data and processes that have so far been excluded and remained 'on paper' - warns Bartolini -: February 9, 2019 is almost upon us, when the serialization obligation, imposed by Directive 2011/62/EU. In other words, a unique serial number will be assigned, connected to individual production data (including product identification, expiry date and lot number, ed) in the form of a two-dimensional matrix barcode”.
A measure decided to strengthen the traceability and safety of medicines on the one hand, and to fight, on the other, the world trade in falsified and illegal medicines, which has increased with the spread of the Internet. Having already equipped itself with a drug tracking system, Italy has been granted a six-year extension by the EU, until 2025. But Italian companies are pressing for alignment with the EU Directive in February 2019: otherwise they will find themselves having to manage a double regime, for the national market and for export, with an unnecessary increase in costs, time and resources.
“In any case – underlines Bartolini – here the reliability of the infrastructures and information systems in the transmission and validation of data will be more than essential: an error in printing, reading or sending the code will be enough to jeopardize not only the product, but also the entire production batch, which will become unsaleable like the single drug”.
To monitor the readiness of companies on this front and highlight the shortcomings that need to be addressed and resolved, a survey was conducted by Afi-Associazione Farmaceutici Industria on a sample of 200 associates between companies and operators in the supply chain at the end of 2017, the results of which will be presented in the autumn in an ad hoc event.
“As a not entirely positive figure – comments Bartolini – the marginal presence of the IT function (5%) should be noted: too modest an impact if one considers what the expected scenario will be from a 4.0 perspective. The answers provided on issues such as system validation, data integrity, ICT infrastructure governance show a situation in which there is much room for improvement. We need a more authoritative support from the top management of companies, to promote a cultural change in management that provides for a transition from the departmental approach to the transversal one (team working), the consolidation of ICT governance processes, and to acquire adequate technological tools and adequately managed able to accommodate the next challenges of the market, starting precisely with traceability and serialization".
On the R&D front, the role of first-in-class drugs (i.e. those that give life to a new class of products) and of "beyond the pill" innovation, which places the drug in continuous interaction with the device, will significantly increase. e-health and other tools. The most frontier activities and projects will then be extended, such as personalized therapies, rare diseases, biotechnologies, gender medicine. Finally, R&D based on the clinical results of drugs will have the opportunity to establish itself: the so-called "Real World Evidence". Commitments that pharmaceutical companies mostly face by entering into collaboration and open innovation agreements with partners from other sectors. Starting with ICT companies, with which to activate synergies in precision medicine starting from the use of Big Data, including biomedical data obtained from wearable devices.
Note: For supply chains means a system of organisations, people, activities, information and resources involved in the process of transferring or providing a product or service from the supplier to the customer. It begins with the raw materials, continues with the creation of the finished product and its warehouse management, and ends with the supply of the final product to the customer. The entire process is divided into various steps, and in each step different professional figures are involved: Marketing; Relations with suppliers; Procurement; Management and storage of raw material stocks; Production; Management and storage of stocks of finished products; Purchase order management; Delivery management; Return logistics.
IoT, an acronym for English internet of things, is a neologism referring to the extension of the Internet to the world of concrete objects and places. Objects (the "things") become recognizable and acquire intelligence thanks to the fact that they can communicate data about themselves and access aggregate information from others. For example, medicine bottles warn family members if they forget to take their medicine. All objects can acquire an active role thanks to the connection to the Net.
There blockchain, in Italian "chain of blocks", can be simplified as a process in which a set of subjects share computer resources (memory, CPU, bandwidth) to make a virtual database available to the community of users. Blockchains are candidates for the role of key application for the Industrial IoT. The technology in question can be used to track billions of connected devices, enabling processing of the transactions they produce and coordination between physical devices.
For augmented reality, or computer mediated reality, means the enrichment of human sensory perception through information, generally manipulated and conveyed electronically, which would not be perceivable with the five senses. It can be used for optimize the realization of a project without the need to physically build a prototype. In this way the company will save time and above all a lot of money.
There 3D printer, new technology used for the production of objects by inserting the appropriate material into the machinery, is seen by many as the beginning of industry 4.0. There fourth industrial revolution. It can be used in any area. 3D printing technology promises considerable savings in the drug production cycle. The FDA has approved a 3D-printed, rapidly disintegrating tablet formulation of the anti-epileptic drug levetiracetam (Spritam). It is the first drug to be produced with 3D printing technology. During manufacturing, the 3D printer dispenses layers of drug powder and a binding agent in liquid form that binds the layers together. Since no compressive forces are used to produce the 3D printed “tablets,” the drug feels solid but porous.





